Decided May 18, 2023
Twitter, Inc. v. Taamneh, No. 21-1496
Gonzalez v. Google LLC, No. 21-1333
Today, a unanimous Supreme Court rejected claims that social-media companies could be held liable under the Anti-Terrorism Act for allegedly not doing “enough” to remove terrorist-related content from their services. In light of that ruling, the Court declined to address whether plaintiffs’ claims were barred by Section 230 of the Communications Decency Act.
Background: Under the Anti-Terrorism Act (ATA) a United States national who is injured by an “act of international terrorism” may recover treble damages. 18 U.S.C. § 2333(a). Victims may also seek recovery from “any person who aids and abets, by knowingly providing substantial assistance, or who conspires with the person who committed such an act of international terrorism.” Id. § 2333(d)(2).
In Twitter, Inc. v. Taamneh, family members of a victim of the 2017 ISIS shooting at the Reina nightclub in Istanbul, Turkey, sued Facebook, Twitter, and Google under the ATA for aiding and abetting the attack. Plaintiffs did not allege that the terrorists who carried out the attack used the companies’ services or that the companies were aware of any specific ISIS accounts tied to the attack. Despite the extensive measures the companies take to block and remove terrorist accounts and terrorist content, plaintiffs alleged that the companies violated the ATA by not doing more.
The district court rejected plaintiffs’ claims, because (1) plaintiffs failed to plausibly allege that the defendants assisted committing the particular attack at issue and (2) it is not enough to allege that the defendants provided general assistance to a terrorist organization. The Ninth Circuit reversed, holding that allegations that a defendant assisted a “broader campaign of terrorism” are enough, even absent allegations that the defendant assisted the particular attack at issue.
Gonzalez v. Google LLC involves substantially similar allegations asserted by family members and the estate of a victim of the 2015 ISIS attacks in Paris. The trial court dismissed plaintiffs’ claims as barred by Section 230 of the Communications Decency Act of 1996, 47 U.S.C. § 230(c)(1), which protects websites and other “interactive computer service” providers from claims based on third-party content, and the Ninth Circuit affirmed.
Issues:
Taamneh: Whether a defendant that provides generic, widely available services to all its numerous users and “regularly” works to detect and prevent terrorists from using those services “knowingly” provided substantial assistance under Section 2333 merely because it allegedly could have taken more “meaningful” or “aggressive” action to prevent such use.
Gonzalez: Whether Section 230 applies to recommendations of third-party content.
Court’s Holdings:
Taamneh: No. Section 2333 requires allegations that the defendant consciously, voluntarily, and culpably participated in the terrorist act at issue in such a way as to help make it succeed.
Gonzalez: Given the overlap with the allegations in Taamneh, the Court declined to address the Section 230 issue and instead remanded for consideration in light of Taamneh.
“[T]he fundamental question of aiding-and-abetting liability [is]: Did defendants consciously, voluntarily, and culpably participate in or support the relevant wrongdoing? … [T]he answer in this case is no.”
Justice Thomas, writing for the Court
Gibson Dunn represented Meta Platforms, Inc. as Respondent Supporting Petitioner in Taamneh and Amicus in Gonzalez.
What It Means:
- In Taamneh, the Court refused to expand aiding-and-abetting liability under Section 2333(d)(2) beyond the traditional, common-law understandings of aiding and abetting.
- Liability under Section 2333(d)(2) is limited to defendants who “consciously and culpably” participate in the specific act of international terrorism that injured the plaintiffs. Although that requirement does not always demand “a strict nexus,” “the more attenuated the nexus, the more courts should demand that plaintiffs show culpable participation though intentional aid that substantially furthered the tort.”
- Today’s opinion also underscores that providing goods or services to the general public should not itself give rise to aiding-and-abetting liability, even if the provider may become aware that its goods or services are being put to illicit ends. As the Court emphasized, imposing liability based on an alleged failure to act requires the plaintiff to make a heightened showing of assistance and scienter.
- The Court also concluded that “[t]he mere creation of” social-media services “is not culpable.”
- The Court’s decision to vacate and remand in Gonzalez without addressing Section 230 returns questions about the scope, interpretation, and application of Section 230 to the courts of appeals, which have developed an extensive body of cases construing and applying Section 230 since the statute was enacted in 1996.
The Court’s opinion is available here and here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
Related Practice: Litigation
| Reed Brodsky +1 212.351.5334 rbrodsky@gibsondunn.com |
Theane Evangelis +1 213.229.7726 tevangelis@gibsondunn.com |
Veronica S. Moyé +1 214.698.3320 vmoye@gibsondunn.com |
| Helgi C. Walker +1 202.887.3599 hwalker@gibsondunn.com |
Related Practice: Media, Entertainment and Technology
| Scott A. Edelman +1 310.557.8061 sedelman@gibsondunn.com |
Kevin Masuda +1 213.229.7872 kmasuda@gibsondunn.com |
Benyamin S. Ross +1 213-229-7048 bross@gibsondunn.com |
Related Practice: Privacy, Cybersecurity and Data Innovation
| Ahmed Baladi +33 (0)1 56 43 13 50 abaladi@gibsondunn.com |
S. Ashlie Beringer +1 650.849.5327 aberinger@gibsondunn.com |
Jane C. Horvath +1 202.955.8505 jhorvath@gibsondunn.com |
| Alexander H. Southwell +1 212.351.3981 asouthwell@gibsondunn.com |
Related Practice: White Collar Defense and Investigations
| Stephanie Brooker +1 201.887.3502 sbrooker@gibsondunn.com |
Nicola T. Hanna +1 213.229.7269 nhanna@gibsondunn.com |
Chuck Stevens +1 415.393.8391 cstevens@gibsondunn.com |
| F. Joseph Warin +1 202.887.3609 fwarin@gibsondunn.com |
Decided May 18, 2023
Andy Warhol Foundation for the Visual Arts v. Goldsmith, No. 21-869
Today, the Supreme Court held 7-2 that the fact that a secondary work of art that incorporates copyrighted source material conveys a distinct meaning or message is not sufficient to render the secondary work transformative for purposes of the fair use analysis.
Background: Photographer Lynn Goldsmith licensed a black and white photograph of Prince to Vanity Fair for use as an artist’s reference in its November 1984 issue. The artist Vanity Fair chose, Andy Warhol, cropped the photograph, silkscreened it onto multiple canvases, and layered each canvas with different brightly colored paints. In all, Warhol created four drawings and 12 silkscreens from the photograph, one of which Vanity Fair ultimately published. After Prince’s death in 2016, the Andy Warhol Foundation licensed one of Warhol’s other silkscreened Prince images to Condé Nast for a special tribute issue. When Goldsmith asserted that Warhol’s image infringed her copyright, the Foundation sued her for a declaration that Warhol’s Prince series was protected under the fair use doctrine. Goldsmith countersued for copyright infringement. The district court held that the images were protected fair use because Warhol transformed Goldsmith’s original photograph to convey a different meaning. The Second Circuit reversed, cautioning that the addition of new meaning was not necessarily transformative.
Issue: Is a work of art sufficiently transformative for purposes of the fair use doctrine when it conveys a different meaning or message from the source material?
Court’s Holding:
No. That a work of art adds a new meaning or message to the source material is not sufficient to render that work transformative—courts must also consider the purpose and commercial nature of both the source material and the secondary work.
“Many secondary works add something new. That alone does not render such uses fair.”
Justice Sotomayor, writing for the Court
What It Means:
- The decision is the first time the Supreme Court has addressed fair use in the context of visual art. The Court addressed only the first fair use factor, namely, “the purpose and character of the use, including whether such use is of a commercial nature or is for nonprofit educational purposes.” 17 U.S.C. § 107(1). For this factor, the Court confirmed that uses that have a further purpose or different character can be “transformative,” but clarified that the degree of difference must be balanced against the commercial nature of the use.
- The Court explained that if the secondary use shares the same or similar purpose as the source material and is of a commercial nature, the factor is likely to weigh against fair use “absent some other justification for copying.” Slip op. 20. For example, the purpose of Warhol’s Soup Can series was “to comment on consumerism rather than advertise soup,” and thus served “a completely different purpose” than the original Campbell’s Soup label. Id. at 27. Here, in contrast, “portraits of Prince used to depict Prince in magazine stories about Prince . . . share substantially the same purpose” and “the copying use is of a commercial nature.” Id. at 12–13.
- The Court limited its holding in Campbell v. Acuff-Rose Music, Inc., 510 U. S. 569 (1994), which upheld fair use in the context of musical parody. The Court explained that Campbell “cannot be read to mean that § 107(1) weighs in favor of any use that adds some new expression, meaning or message.” Slip op. 28. By limiting the availability of the fair use defense for secondary works that merely claim some further purpose or different character, the Court thus placed a premium on incentivizing and protecting original creation.
- The Court’s decision could create new avenues to allege infringement by secondary works that build on or reference other works, although the Court emphasized that its analysis was “limit[ed]” to Warhol’s “commercial licensing of Orange Prince to Condé Nast.” Slip op. 21.
The Court’s opinion is available here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
Related Practice: Intellectual Property
| Kate Dominguez +1 212.351.2338 kdominguez@gibsondunn.com |
Y. Ernest Hsin +1 415.393.8224 ehsin@gibsondunn.com |
|
| Josh Krevitt +1 212.351.4000 jkrevitt@gibsondunn.com |
Jane M. Love, Ph.D. +1 212.351.3922 jlove@gibsondunn.com |
Related Practice: Fashion, Retail and Consumer Products
| Howard S. Hogan +1 202.887.3640 hhogan@gibsondunn.com |
Decided May 18. 2023
Amgen Inc. et al. v. Sanofi et al., No. 21-757
Today, the Supreme Court unanimously held that the Patent Act’s enablement requirement is satisfied only when a patent’s specification allows persons skilled in the art to make and use the full scope of the invention without more than a “reasonable” amount of experimentation under the circumstances.
Background: Amgen and Sanofi produce antibody medications to treat high LDL cholesterol. In 2011, each party obtained a patent covering the specific antibody used in its drugs. The antibodies in the drugs work by preventing a protein from interfering with the body’s natural regulation of LDL cholesterol. In 2014, Amgen obtained two patents that covered not only 26 specifically listed antibodies by their amino acid sequences, but also the “entire genus” of antibodies that performs this function—a claim that arguably covers millions of antibodies. Amgen then sued Sanofi for patent infringement.
Sanofi argued that the relevant claims were invalid because they did not satisfy the enablement requirement of the Patent Act, which requires a patent specification to describe “the manner and process of making and using” the invention in such a way “as to enable any person skilled in the art to which it pertains . . . to make and use the same.” 35 U.S.C. § 112(a). According to Sanofi, the claims for the antibodies beyond the 26 specifically listed essentially required scientists to engage in a trial-and-error process of discovery. After lengthy proceedings, the district court agreed, and the Federal Circuit affirmed.
Issue: Where a patent claims an entire class of processes, machines, manufactures, or compositions of matter, must the patent specification enable a person skilled in the art to make and use the entire class?
Court’s Holding:
Yes. To satisfy the Patent Act’s enablement requirement, a patent’s specification must enable the full scope of the invention as defined by the patent’s claims, subject to a reasonable amount of adaptation or experimentation.
“[T]he specification must enable the full scope of the invention as defined by its claims. The more one claims, the more one must enable.”
Justice Gorsuch, writing for the Court
What It Means:
- The Patent Act’s enablement requirement is not satisfied when a patent claims a broad class but its specification requires undue experimentation or trial-and-error discovery to make and use the entire class.
- The Court stopped short of requiring that a patent specification need always describe with particularity how to make and use every embodiment within a claimed class. In some cases, it may be sufficient to provide an example that discloses a general quality running through the class.
- The Court stated that a specification is not necessarily inadequate simply because it involves some measure of adaptation or testing, but such experimentation must be reasonable. Reasonableness will depend on the nature of the invention and the underlying art, meaning that courts will likely make this determination on a case-by-case basis.
- Overall, the decision reinforces the enablement requirement as a defense to patent-infringement claims, and will likely incentivize more detailed specifications in patent applications.
The Court’s opinion is available here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
Related Practice: Intellectual Property
| Kate Dominguez +1 212.351.2338 kdominguez@gibsondunn.com |
Y. Ernest Hsin +1 415.393.8224 ehsin@gibsondunn.com |
|
| Josh Krevitt +1 212.351.4000 jkrevitt@gibsondunn.com |
Jane M. Love, Ph.D. +1 212.351.3922 jlove@gibsondunn.com |
Unfair business practices encompass fraud, misrepresentation, and oppressive or unconscionable acts or practices by businesses, often against consumers. In California, individuals and specified governmental agencies are authorized to bring civil actions for unfair competition and to recover civil penalties or injunctive relief pursuant to the Unfair Competition Law (UCL) under Business and Professions Code Section 17200.
California’s Government Code authorizes the Attorney General, as a head of a state department, to investigate and prosecute actions concerning certain matters, including UCL violations. It also equips the Attorney General with certain investigatory tools, including pre-litigation subpoena power, to effectuate enforcement of the law.
Among the agencies authorized to prosecute UCL actions are city attorneys of cities with populations in excess of 750,000 and county counsel of any county within which a city has a population in excess of 750,000, as well as (in the case of San Francisco) city attorneys of a city and county. Although certain county counsel and city attorneys can bring UCL actions, prior to the passage and enactment of AB 2766, these entities were not afforded the same tools as the Attorney General and district attorneys to investigate possible unfair competition cases. AB 2766, enacted on January 1, 2023, amended Section 16759 of the Business and Professions Code to extend these same investigatory powers to city and county attorneys who are also authorized to bring UCL claims (subject to certain requirements).
Sponsors and supporters of the bill cited increased reports of consumer fraud and price gouging during the COVID-19 pandemic, which they claimed demonstrated a need for greater enforcement of California’s consumer protection laws.
Pre-Existing Relevant Law
There is a constellation of statutes that are relevant to enforcement of the UCL.
Business and Professions Code Section 17200 defines “unfair competition” to include any unlawful, unfair, or fraudulent business act or practice and any unfair, deceptive, untrue, or misleading advertising, and any act prohibited by the False Advertising Law, Business and Professions Code Section 17500 et seq. Accordingly, the UCL is a tool for enforcement relating to a wide range of consumer-facing business activity.
Government Code Section 11181 authorizes the heads of each state department to make investigations and prosecute actions concerning matters relating to the business activities and subjects under their jurisdiction; violations of any law or rule or order of the department; and such other matters as may be provided by law. In order to effectuate these investigations and actions, the law provides the heads of these departments with certain investigatory powers. Among these powers is the ability to promulgate interrogatories; the ability to issue subpoenas for the attendance of witnesses and the production of certain documents, testimony, or other materials, and the ability to inspect and copy those same documents and materials. With regard to the UCL specifically, the relevant “state department head” is the Attorney General.
But the Attorney General is not the only entity authorized to prosecute violations of the UCL. Under Business and Professions Code Section 17204, in addition to the Attorney General, actions under the UCL may be brought by a district attorney, a city attorney or county counsel, or an individual person who has suffered injury in fact and has lost money or property as a result of the unfair competition. In the case of city attorneys and county counsels of counties and cities with populations smaller than 750,000, consent from the district attorney is required to bring an action under the UCL. In counties and cities with populations larger than that number, no such consent is required. Thus, the only city attorneys with authority to independently bring actions under the UCL are those in San Jose, San Diego, and Los Angeles, and the only county counsel are those in San Diego County, Los Angeles County, and Santa Clara County (as the cities of San Diego, Los Angeles, and San Jose all have populations over 750,000).
In order to facilitate their investigation of violations of the UCL, district attorneys are granted the same investigative powers given to the Attorney General pursuant to Section 16759 of the Business and Professions Code, subject to certain safeguards. In particular, district attorneys’ investigations under this section must abide by the procedures laid out in the relevant sections of Government Code and are subject to the California Right to Financial Privacy Act.
Changes to the Law Under AB 2766
AB 2766 extended the same investigatory powers granted to the Attorney General and the district attorneys to the city attorneys and county counsel which are already authorized to bring UCL claims when these entities reasonably believe that there may have been a violation of the UCL.
Specifically, the new law:
- Grants all of the powers that are granted to the Attorney General as the head of a state department to make investigations and prosecute actions regarding unfair competition laws (commencing with Business and Professions Code Section 17200) to the city attorney of any city having a population in excess of 750,000, to the county counsel of any county within which a city has a population in excess of 750,000, or to a city attorney of a city and county, when the city attorney or county counsel reasonably believes that there may have been a violation of the unfair competition laws;
- Makes any action brought by a city attorney or county counsel pursuant to the bill, like an action brought by the Attorney General or district attorney, subject to the provisions of the “California Right to Financial Privacy Act” set forth in existing law; and
- Clarifies that court orders sought pursuant to the bill shall be sought in the superior court of the county in which the district attorney, city attorney, or county counsel, who is seeking the order and authorized to bring an action pursuant to the bill, holds office.
AB 2766 amended Business and Professions Code section 16759—which previously provided district attorneys with pre-litigation investigatory authority for potential UCL actions—to expressly provide city attorneys and county counsel in large jurisdictions with the same pre-litigation investigative authority for suspected UCL violations. Based on the law’s population requirements, AB 2677 applies to legal authorities in San Diego City and County, Los Angeles City and County, Santa Clara County and San Jose, and San Francisco (which co-sponsored the bill).
Arguments For And Against AB 2766
AB 2766 garnered substantial support on both the Senate and Assembly floors (29 in favor versus 9 against and 57 in favor versus 15 against, respectively). This section will detail some highlights of the discourse regarding the bill in the Legislature prior to its passage.
Proponents of the law argued that “AB 2766 will bolster consumer protection enforcement efforts” and will “[e]nsur[e] a robust consumer protection investigatory framework to protect businesses that play by the rules.” Further, it will “ensure consistency in the UCL for those empowered to enforce [it].”
Opponents argued that the bill—particularly the subpoena power—”potentially infring[es] on the judicial due process rights of businesses, organizations and individuals in California,” and “makes businesses vulnerable to baseless fishing expeditions and political maneuvers, as standard necessary (sic) to issue a pre-litigation subpoena is disturbingly low.”
Supporters of the bill claimed that opponents’ concerns about overreaching were unfounded, as “important safeguards exist under current law to protect against overreach by a prosecutor,” which also apply under AB 2766. Specifically, the city attorneys and county counsels with new investigative authority are subject to the same parameters currently applied to district attorneys’ use of these investigatory powers in Section 16759, including the procedures laid out in the Government Code, and will also be subject to the California Right to Financial Privacy Act. This Act protects the confidential relationship between financial institutions and their customers by, in part, providing more procedural safeguards with respect to subpoenaing financial records. In addition, these city attorneys and county counsel are only granted these expanded investigatory powers when the city attorney or county counsel “reasonably believes that there may have been a violation of [the UCL].” Further, the recipient of the subpoena can refuse to comply, leaving it up to the prosecutor to go to court to compel production.
Potential Impacts of AB 2766
Proponents of AB 2766 claimed that complaints of UCL violations rose during the pandemic, necessitating the bolstering of UCL enforcement measures. In response, Assembly Member Brian Maienschein (D-San Diego) authored the bill after “work[ing] with numerous attorneys to identify solutions to strengthening consumer protection laws in California.” The bill was co-sponsored by the City and County of San Francisco, City of San Diego, County of Los Angeles, and County of Santa Clara.
Exactly how widely the new powers granted under the bill will be operationalized remains to be seen, but there are many indications from attorneys in the co-sponsoring cities and counties that they intend to use them widely. Following Governor Gavin Newsom’s signing of AB 2766 in September 2022, many public prosecutors lauded the legislation and publicly forecasted their offices’ plans to use the new investigative powers once the law took effect. Said San Francisco City Attorney David Chiu:
“During the pandemic we saw a troubling surge in price gouging, consumer fraud, and unfair business practices,” said San Francisco City Attorney David Chiu. “As our office continues to pursue bad actors that seek to defraud the public, this new law will give us more tools to better protect consumers and workers.”
Then-Los Angeles City Attorney Mike Feuer echoed this sentiment, stating:
“Time and again, we’ve successfully fought for hard-working Angelenos who’ve been ripped off—sometimes devastated—by unlawful business practices. Our office will be all the more impactful now that we have this key investigative tool, allowing us to get to the heart of scams and put a stop to them even faster.”
Acting Los Angeles County Counsel Dawyn Harrison, Santa Clara County Counsel James R. Williams, and San Diego City Attorney Mara W. Elliott also released similar statements.
Indeed, the San Francisco and San Diego City Attorneys’ offices have already begun utilizing their investigative powers in a highly public context—openly touting their initiation of an investigation into a home title locking business, which the city attorneys allege to be deceptive and predatory. Not only did the San Francisco City Attorney issue a press release proudly proclaiming that “[t]he subpoena [it issued] reflects an early use of city attorney’s authority under Assembly Bill 2766”–it coupled the announcement with a clear indication that the office was seeking to use its new power immediately to stop, and not just investigate, the target’s conduct, which the office labeled as “a scam, plain and simple.”
It is impossible to say with certainty that AB 2766 will result in increased numbers of UCL prosecutions by public prosecutors, as county counsel have possessed the power to prosecute UCL violations since the passage of SB 709 in 1991 and city attorneys since the passage of SB 1725 in 1974. However, AB 2766 is aligned with a general push toward broader power to prosecute and enforce the UCL since the advent of the statute. Most recently, the Supreme Court of California confirmed that local prosecutors’ power to enforce the UCL goes beyond their territorial jurisdictions, in lockstep with that of the Attorney General. (Abbott Lab’ys v. Superior Ct. of Orange Cnty. (2020) 9 Cal. 5th 642, 661.) Because of the relationship between Sections 17200 and 16759 of the Business and Professions Code, the new investigatory powers of city and county attorneys under AB 2766 also likely extend outside of local prosecutors’ jurisdictions, granting them the authority to investigate conduct that occurred elsewhere. This combination of greater authority to investigate UCL offenses, more expansive jurisdictional reach, and early signals from newly empowered city and county attorneys following the passage of AB 2766, point to the potential for a pronounced rise in aggressive UCL investigations by public prosecutors—particularly by city attorneys in California’s largest cities, which are already known for their frequent use of affirmative enforcement lawsuits on behalf of consumers.
The following Gibson Dunn lawyers prepared this client alert: Winston Chan, Michael Farhang, Douglas Fuchs, Nicola T. Hanna, Meredith Spoto, Chuck Stevens, Eric D. Vandevelde, Benjamin Wagner, and Debra Wong Yang.
Gibson Dunn has more than 250 white collar lawyers around the globe who are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any member of the firm’s U.S. White Collar Defense and Investigations or Anti-Corruption and FCPA practice groups below:
White Collar Defense and Investigations Group – United States:
Los Angeles
Michael H. Dore (+1 213-229-7652, mdore@gibsondunn.com)
Michael M. Farhang (+1 213-229-7005, mfarhang@gibsondunn.com)
Diana M. Feinstein (+1 213-229-7351, dfeinstein@gibsondunn.com)
Douglas Fuchs (+1 213-229-7605, dfuchs@gibsondunn.com)
Nicola T. Hanna – Co-Chair (+1 213-229-7269, nhanna@gibsondunn.com)
Poonam G. Kumar (+1 213-229-7554, pkumar@gibsondunn.com)
Marcellus McRae (+1 213-229-7675, mmcrae@gibsondunn.com)
Eric D. Vandevelde (+1 213-229-7186, evandevelde@gibsondunn.com)
Debra Wong Yang (+1 213-229-7472, dwongyang@gibsondunn.com)
Meredith K. Spoto (+1 213-229-7060, mspoto@gibsondunn.com)
San Francisco
Winston Y. Chan (+1 415-393-8362, wchan@gibsondunn.com)
Charles J. Stevens – Co-Chair (+1 415-393-8391, cstevens@gibsondunn.com)
Michael Li-Ming Wong (+1 415-393-8333, mwong@gibsondunn.com)
Palo Alto
Benjamin Wagner (+1 650-849-5395, bwagner@gibsondunn.com)
Washington, D.C.
Stephanie Brooker – Co-Chair (+1 202-887-3502, sbrooker@gibsondunn.com)
Courtney M. Brown (+1 202-955-8685, cmbrown@gibsondunn.com)
David P. Burns (+1 202-887-3786, dburns@gibsondunn.com)
John W.F. Chesley (+1 202-887-3788, jchesley@gibsondunn.com)
Daniel P. Chung (+1 202-887-3729, dchung@gibsondunn.com)
M. Kendall Day (+1 202-955-8220, kday@gibsondunn.com)
David Debold (+1 202-955-8551, ddebold@gibsondunn.com)
Michael S. Diamant (+1 202-887-3604, mdiamant@gibsondunn.com)
Gustav W. Eyler (+1 202-955-8610, geyler@gibsondunn.com)
Richard W. Grime (+1 202-955-8219, rgrime@gibsondunn.com)
Scott D. Hammond (+1 202-887-3684, shammond@gibsondunn.com)
George J. Hazel (+1 202-887-3674, ghazel@gibsondunn.com)
Judith A. Lee (+1 202-887-3591, jalee@gibsondunn.com)
Adam M. Smith (+1 202-887-3547, asmith@gibsondunn.com)
Patrick F. Stokes – Co-Chair (+1 202-955-8504, pstokes@gibsondunn.com)
Oleh Vretsona (+1 202-887-3779, ovretsona@gibsondunn.com)
F. Joseph Warin – Co-Chair (+1 202-887-3609, fwarin@gibsondunn.com)
Amy Feagles (+1 202-887-3699, afeagles@gibsondunn.com)
David C. Ware (+1 202-887-3652, dware@gibsondunn.com)
Ella Alves Capone (+1 202-887-3511, ecapone@gibsondunn.com)
Nicholas U. Murphy (+1 202-777-9504, nmurphy@gibsondunn.com)
Melissa Farrar (+1 202-887-3579, mfarrar@gibsondunn.com)
Nicole Lee (+1 202-887-3717, nlee@gibsondunn.com)
Jason H. Smith (+1 202-887-3576, jsmith@gibsondunn.com)
Pedro G. Soto (+1 202-955-8661, psoto@gibsondunn.com)
New York
Zainab N. Ahmad (+1 212-351-2609, zahmad@gibsondunn.com)
Reed Brodsky (+1 212-351-5334, rbrodsky@gibsondunn.com)
Mylan L. Denerstein (+1 212-351-3850, mdenerstein@gibsondunn.com)
Barry R. Goldsmith (+1 212-351-2440, bgoldsmith@gibsondunn.com)
Karin Portlock (+1 212-351-2666, kportlock@gibsondunn.com)
Mark K. Schonfeld (+1 212-351-2433, mschonfeld@gibsondunn.com)
Orin Snyder (+1 212-351-2400, osnyder@gibsondunn.com)
Alexander H. Southwell (+1 212-351-3981, asouthwell@gibsondunn.com)
Brendan Stewart (+1 212-351-6393, bstewart@gibsondunn.com)
Denver
Kelly Austin – Co-Chair (+1 303-298-5980, kaustin@gibsondunn.com)
Ryan T. Bergsieker (+1 303-298-5774, rbergsieker@gibsondunn.com)
Robert C. Blume (+1 303-298-5758, rblume@gibsondunn.com)
John D.W. Partridge (+1 303-298-5931, jpartridge@gibsondunn.com)
Laura M. Sturges (+1 303-298-5929, lsturges@gibsondunn.com)
Houston
Gregg J. Costa (+1 346-718-6649, gcosta@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
Gibson Dunn is pleased to announce the establishment of its Child and Forced Labor Risks Global Task Force to help our clients prevent illegal child and forced labor in their workforce, evaluate their supply chains, and respond to investigations and litigation arising from illegal child labor and forced labor allegations.
Governments around the world are increasing their scrutiny of illegal child labor and forced labor. In the United States, the Department of Labor and Congress have recently launched investigations of companies’ child labor policies and compliance practices that pose substantial legal, financial, and reputational risks for those companies. Congress also has enacted statutes to prevent forced labor globally and recently questioned several companies regarding allegations of forced labor in their supply chains. In Europe, laws combating modern slavery and child labor are emerging across individual jurisdictions, with potential for additional legislation that will impose mandatory human rights and due diligence obligations on certain corporations with a European commercial footprint. Similarly, governments in the Asia Pacific region are grappling with ways to address the issue through a combination of legislation and enforcement.
The Gibson Dunn team offers holistic compliance and response strategies to help our clients implement best-of-class policies related to forced labor and illegal child labor and to ensure they are prepared to respond effectively to civil, criminal, or congressional investigations or litigation relating to alleged forced labor or illegal child labor in their workforce or their supply chains.
Working together, our Labor and Employment, White Collar Defense and Investigations, Congressional Investigations, Crisis Management, Public Policy, Environmental Social Governance (ESG), Securities Regulation and Corporate Governance, Transnational Litigation, International Trade, and Litigation practice groups provide a range of services to root out and prevent forced labor and illegal child labor in clients’ workforce and help clients emerge stronger after investigations and enforcement actions.
This document is for informational purposes only and does not, and is not intended to, constitute legal advice or create an attorney-client relationship. You should contact a Gibson Dunn attorney directly to see if they are able to provide legal advice with respect to a particular legal matter.
Gibson Dunn’s multidisciplinary Child Forced Labor Risks Global Task Force (ChildLaborTaskForce@gibsondunn.com) members are available to assist clients in their efforts to prevent illegal child labor in their own and their suppliers’ workforce and to guide clients through government investigations and litigation based on allegations of illegal child labor or forced labor. Please contact the Gibson Dunn lawyer with whom you usually work, the Task Force, or any of the following authors for additional information about how we may assist you.
Congressional Investigations and Public Policy:
Michael D. Bopp – Washington, D.C. (+1 202-955-8256, mbopp@gibsondunn.com)
Roscoe Jones, Jr. – Washington, D.C. (+1 202-887-3530, rjones@gibsondunn.com)
Amanda H. Neely – Washington, D.C. (+1 202-777-9566, aneely@gibsondunn.com)
Environmental Social Governance (ESG):
Susy Bullock – London (+44 (0) 20 7071 4283, sbullock@gibsondunn.com)
Perlette Michèle Jura – Los Angeles (+1 213-229-7121, pjura@gibsondunn.com)
Robert Spano – London (+44 (0) 20 7071 4902, rspano@gibsondunn.com)
International Trade:
Adam M. Smith – Washington, D.C. (+1 202-887-3547, asmith@gibsondunn.com)
Christopher T. Timura – Washington, D.C. (+1 202-887-3690, ctimura@gibsondunn.com)
Labor and Employment:
Eugene Scalia – Washington, D.C. (+202-955-8210, escalia@gibsondunn.com)
Jason C. Schwartz – Washington, D.C. (+1 202-955-8242, jschwartz@gibsondunn.com)
Katherine V.A. Smith – Los Angeles (+1 213-229-7107, ksmith@gibsondunn.com)
Litigation and Transnational Litigation:
Perlette Michèle Jura – Los Angeles (+1 213-229-7121, pjura@gibsondunn.com)
William E. Thomson – Los Angeles (+1 213-229-7891, wthomson@gibsondunn.com)
Securities Regulation and Corporate Governance:
Lori Zyskowski – New York (+1 212-351-2309, lzyskowski@gibsondunn.com)
White Collar Defense and Investigations:
Michael S. Diamant – Washington, D.C. (+1 202-887-3604, mdiamant@gibsondunn.com)
Oliver D. Welch – Hong Kong (+852 2214 3716, owelch@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
On May 12, 2023, the German lawmaker passed a law protecting those who report violations around the workplace (the “Whistleblower Protection Act”, or “Act”). As an essential part of the Act, companies with 50 employees or more in Germany must establish internal reporting channels for this purpose.
I. Background
The new law implements the EU Whistleblower Directive 2019/1937 (“Directive”), which was due to be transformed into national law by December 17, 2021. Like most other EU member states, Germany was late in implementing the law.[1]
With regard to the scope, the German lawmaker goes far beyond the Directive in that almost all kinds of violations can be subject to protected whistleblowing. This even includes actions that are not illegal, but deemed to be an “abuse”, because they are directed against the “purpose” of legal provisions.
II. Main Obligations
Under the Act, there are three kinds of whistleblowing: (i) internal reporting within an organization, (ii) external reporting to a special government agency, and (iii) public disclosure. The latter is only permissible if external reporting has not proven successful or in several other cases, i.e., if there is an urgent threat to public interests. Unfortunately, as mandated by the Directive, the Act does not stipulate a priority of internal over external reporting. However, employees are explicitly enhanced to do so, and employers are supposed to promote internal over external reporting.
Companies with 50 or more employees in Germany have to offer internal reporting lines for whistleblowers and set up properly (yet not necessarily full-time) staffed functions to deal with such reports. The company can outsource such tasks to an external partner or – as will often be the case – defer to its centralized group reporting scheme as long as the local entity remains responsible for remediation measures.[2] There is a transition rule for companies between 50 and 249 employees: Their obligation to set up internal reporting lines is deferred until December 17, 2023.
Neither internal nor external reporting lines have to provide for anonymous reports, but should handle them nevertheless.
III. Identity Protection, Non-Retaliation
The internal reporting cell has to protect (i.e. not disclose) the identity of good-faith whistleblowers, not even to the company’s management, unless the whistleblower consents or a public authority asks for it. The person being subject to the report enjoys a similar, yet weaker disclosure protection.
Whistleblowers acting in good faith must not be retaliated against in any way because of their report. Such retaliation could consist in, e.g., dismissal, pay cut, relocation, or other disadvantages by the employer.
If there has been a retaliation against the whistleblower, they can claim damages from the parties responsible for the retaliation (typically the employer).
In order to help the whistleblower procedurally, the Act presumes that any disadvantage after the report is presumed to be retaliation. This presumption can be rebutted, and employers should carefully document their personnel measures against whistleblowers in order to be able to prove that the measure is based on other reasons than the whistleblowing.[3]
Finally, good-faith whistleblowers shall not be legally liable for retrieving the information they report, unless the access or use of said information was a criminal act in itself. Even trade secrets may be disclosed, if it is necessary to lance the report.
IV. Companies’ Responses and Next Steps
Any company with 50 employees or more in Germany now has to check whether they have adequate reporting lines in place and properly staffed functions to handle whistleblower reports. Companies with 50 to 249 employees do not have to install the reporting lines until December 17, 2023.
Other than the Directive, the German lawmaker expressly acknowledges centralized reporting lines to be in line with the Act. This is good news for multinational organizations, after the EU Commission fervently contested that such centralized systems were in line with the Directive. It remains to be seen whether the EU Commission accepts those local laws that allow centralized reporting lines.
Multinational organizations operating companies with more than 50 entities in multiple EU member states are well advised to assess the requirements of the respective local implementation laws. In light of the leeway granted to the EU member states in implementing the Directive, individual provisions may vary significantly across the EU member states.
HR departments should carefully prepare and document any measure against employees that might be perceived as retaliation in case the employees have launched a whistleblower report. If the employers can provide sound reasons for their decision, they should be able to rebut the statutory presumption contained in the Act.
Violations of the obligations contained in the Act carry a fine of up to € 50,000.
__________________________
[1] See https://www.whistleblowingmonitor.eu/ for an overview of the implementation status across the EU members states.
[2] See https://www.gibsondunn.com/wp-content/uploads/2022/02/Zimmer-Humphrey-Petzen-Ja-bitte-Meldesysteme-nach-der-Whistleblower-Richtlinie-der-EU-Betriebs-Berater-02-2022.pdf.
[3] See https://www.gibsondunn.com/hilfe-fur-hinweisgeber-beweislastumkehr-nach-%c2%a7-36-ii-hinschg-rege/.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. If you wish to discuss any of the matters set out above, please contact the Gibson Dunn lawyer with whom you usually work, any member of Gibson Dunn’s Labor and Employment or White Collar Defense and Investigations teams in Germany, or the following authors in Munich:
Katharina Humphrey (+49 89 189 33 217, khumphrey@gibsondunn.com)
Mark Zimmer (+49 89 189 33 230, mzimmer@gibsondunn.com)
Corporate Compliance / White Collar Matters
Ferdinand Fromholzer (+49 89 189 33 270, ffromholzer@gibsondunn.com)
Kai Gesing (+49 89 189 33 285, kgesing@gibsondunn.com)
Markus Nauheim (+49 89 189 33 222, mnauheim@gibsondunn.com)
Markus Rieder (+49 89 189 33 260, mrieder@gibsondunn.com)
Benno Schwarz (+49 89 189 33 210, bschwarz@gibsondunn.com)
Finn Zeidler (+49 69 247 411 530, fzeidler@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
On May 12, 2023, the IRS and Treasury issued Notice 2023-38 (the “Notice”) (here), which provides initial guidance for developers and investors seeking to qualify projects for the domestic content bonus credit available under sections 45, 45Y, 48, and 48E (the “Domestic Content Bonus Credit”).[1] Although not explicit, the Notice also provides guidance regarding the receipt of full “direct pay” amounts for projects beginning construction after 2023.
The Inflation Reduction Act of 2022 (the “IRA”)[2] provided for an enhanced or “bonus” credit in respect of certain qualified facilities, energy projects, and energy storage technologies if a taxpayer certifies that the steel, iron, or manufactured products that are components of such a facility, project or technology were produced in the United States (the “Domestic Content Requirement”).[3] The IRA also made credits under sections 45, 45Y, 48 and 48E refundable for certain tax-exempt entities.[4] Beginning in 2024, however, these refunds (so-called “direct pay”) are subject to phaseout for projects that do not meet the Domestic Content Requirement; specifically, a 10-percent haircut applies to projects begun in 2024, a 15-percent haircut for projects begun in 2025, and projects begun in 2026 and after would be wholly ineligible for refunds, in each case unless the IRS makes an exception to the requirement.[5]
The Notice provides rules on which taxpayers may, subject to limitations discussed below, rely for determining whether projects will meet the Domestic Content Requirement. Before providing a number of practical observations regarding the guidance in the Notice, the Alert covers the following:
- Background. Increased tax credits are available with respect to certain energy generation (e.g., wind, solar) and storage projects that satisfy the Domestic Content Requirement.
- Domestic Content Requirement.
- Manufactured Products vs. Steel/Iron Components. The Domestic Content Requirement applies differently for (i) manufactured products and (ii) steel or iron components of a project. The Notice provides helpful guidance for determining whether a particular component is a manufactured product or a steel or iron component, including a useful safe harbor that applies to certain types of projects.
- Determining Whether Domestic Content Requirement Satisfied. Once a component has been categorized as either a manufactured product or a steel or iron component, the Notice provides guidance for determining whether the Domestic Content Requirement is satisfied with respect to the relevant project. The rules for manufactured products are particularly complicated and may be challenging to satisfy.
- Certification and Substantiation. In addition to meeting substantive requirements, taxpayers seeking to satisfy the Domestic Content Requirement must meet detailed certification and substantiation requirements.
Background
A taxpayer is eligible to claim a Domestic Content Bonus Credit in respect of projects that meet the Domestic Content Requirement under sections 45 and 45Y (the “PTC”) and sections 48 and 48E (the “ITC”) if the taxpayer timely certifies to the IRS that the applicable requirements have been satisfied. For PTC projects, if the Domestic Content Bonus Credit is available, the amount of the section 45 or 45Y credit is increased by a maximum of 10 percent, and for ITC projects, the amount of the section 48 or 48Y credit is increased by 10 percentage points.[6]
Under current law, the PTC is claimed in respect of the production of electricity from qualified energy resources (e.g., wind and solar) at a qualified facility during the 10-year period beginning on the date on which the project was placed in service. For zero-emission energy projects that begin construction after 2024, the PTC will transition to a new technology-neutral credit under section 45Y . The current ITC is claimable in respect of the basis of certain energy property (e.g., wind, solar, and energy storage property). Like the PTC, for zero-emission energy projects that begin construction after 2024, the ITC will transition to a new technology-neutral ITC under section 48E.
Domestic Content Requirement
The Domestic Content Requirement applies differently with respect to two different categories of components: (1) steel or iron components, which are subject to a more stringent test, and (ii) “Manufactured Products” (defined as any item produced as a result of a manufacturing process).[7]
Application of the Domestic Content Requirement is a two-step process:
- In the first step, each article, material, or supply that is directly incorporated into a project (each, a “Project Component”) is categorized to determine whether that Project Component must meet either the Steel or Iron Requirement or the Manufactured Products Requirement (each as defined below).
- In the second step, each Project Component is analyzed to determine whether it satisfies the Steel or Iron Requirement or the Manufactured Products Requirement, as applicable.
Step one is applied by first analyzing Project Components that are made primarily of steel or iron. If a steel or iron Project Component is both (i) a construction material and (ii) “structural in function” (e.g., towers (wind facilities) or photovoltaic module racking (solar facilities)), the component is subject to the Steel or Iron Requirement. The Notice provides a non-exhaustive list of steel or iron items that are not “structural in function” (and therefore not subject to the Steel or Iron Requirement): nuts, bolts, screws, washers, cabinets, covers, shelves, clamps, fittings, sleeves, adapters, tie wire, spacers and door hinges. Any Project Components that are Manufactured Products (i.e., those Project Components that are not steel or iron Project Components and that underwent a manufacturing process) are subject to the Manufactured Products Requirement.
In a very welcome development, the Notice provides a safe harbor for applying step one to certain identified and commonly analyzed Project Components. The list of Project Components covers only limited categories of projects and does not include all Project Components that may comprise those projects. These classifications nevertheless provide helpful guidance that should permit taxpayers to make strategic sourcing decisions pending the publication of regulations. These safe harbor classifications are outlined in Table 2 of the Notice, which is reproduced immediately below.
|
Applicable Project |
Applicable Project Component |
Categorization |
|
Utility-scale photovoltaic system |
Steel photovoltaic module racking |
Steel/Iron |
|
Pile or ground screw |
Steel/Iron |
|
|
Steel or iron rebar in foundation (e.g., concrete pad) |
Steel/Iron |
|
|
Photovoltaic tracker |
Manufactured Product |
|
|
Photovoltaic module (which includes the following Manufactured Product Components, if applicable: photovoltaic cells, mounting frame or backrail, glass, encapsulant, backsheet, junction box (including pigtails and connectors), edge seals, pottants, adhesives, bus ribbons, and bypass diodes) |
Manufactured Product |
|
|
Inverter |
Manufactured Product |
|
|
Land-based wind facility |
Tower |
Steel/Iron |
|
Steel or iron rebar in foundation (e.g., spread footing) |
Steel/Iron |
|
|
Wind turbine (which includes the following Manufactured Product Components, if applicable: the nacelle, blades, rotor hub, and power converter) |
Manufactured Product |
|
|
Wind tower flanges |
Manufactured Product |
|
|
Offshore wind facility |
Tower |
Steel/Iron |
|
Jacket foundation |
Steel/Iron |
|
|
Wind tower flanges |
Manufactured Product |
|
|
Wind turbine (which includes the following Manufactured Product Components, if applicable: the nacelle, blades, rotor hub, and power converter) |
Manufactured Product |
|
|
Transition piece |
Manufactured Product |
|
|
Monopile |
Manufactured Product |
|
|
Inter-array cable |
Manufactured Product |
|
|
Offshore substation |
Manufactured Product |
|
|
Export cable |
Manufactured Product |
|
|
Battery energy storage technology |
Steel or iron rebar in foundation (e.g., concrete pad) |
Steel/Iron |
|
Battery pack (which includes the following Manufactured Product Components, if applicable: cells, packaging, thermal management system, and battery management system) |
Manufactured Product |
|
|
Battery container/housing |
Manufactured Product |
|
|
Inverter |
Manufactured Product |
Once each Project Component has been categorized at step one, in the second step each Project Component is analyzed to determine whether it satisfies the Steel or Iron Requirement or the Manufactured Products Requirement.
Steel or Iron Requirement
The “Steel or Iron Requirement” is satisfied with respect to a Project Component if all manufacturing processes with respect to the Project Component (other than metallurgical processes involving refinement of steel additives) take place in the United States.[8]
Manufactured Products Requirement
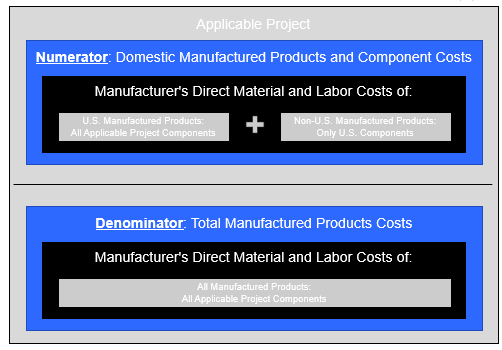
The “Manufactured Products Requirement” is satisfied if a statutory percentage (ranging from 20 percent to 55 percent, as discussed below) of the total costs of the Project Components that are Manufactured Products are attributable to (i) “U.S. Manufactured Products” or (ii) “U.S. Components” (each as defined below).[9]
Application of the Manufactured Products Requirement is a five-step process:
- First, each Project Component that is a Manufactured Product must be separated into those Project Components for which all of the manufacturing processes take place in the United States, and those Project Components that do not.
- Second, each Project Component must be separated into its individual direct components. A component that is “directly” incorporated into a Project Component is referred to in the Notice as a “Manufactured Product Component.” The safe harbor in Table 2 of the Notice (reproduced above) lists certain “Manufactured Product Components” of specified Manufactured Products.
- Third, for Project Components manufactured in the United States as determined at step one, it must be determined whether each “Manufactured Product Component” of such Project Component is “of U.S. origin” (in the case of manufactured components, “regardless of the origin of its subcomponents”). Project Components satisfying step 3 are “U.S. Manufactured Products.”
- Fourth, for Project Components that are not U.S. Manufactured Products, it must be determined which (if any) “Manufactured Product Components” of such Project Component are mined, produced, or manufactured in the United States. Any such Manufactured Product Components are “U.S. Components.”
- Fifth, and finally, the costs of the U.S. Manufactured Products and the U.S. Components for the project must be divided by the total cost of the Project Components that are Manufactured Products to reach a percentage that is compared to the applicable statutory percentage (discussed below). If the percentage determined at step 5 equals or exceeds the applicable statutory percentage, the project satisfies the Manufactured Products Requirement.
In computing the “costs” included in the numerator and the denominator of the fraction at step 5, only direct material costs and labor costs that were paid or incurred by the manufacturer (i.e., the person that performed the manufacturing process that produced the relevant component or product) are included.[10] In computing the cost of any U.S. Component that is incorporated into a Manufactured Product that also includes Manufactured Product Components not manufactured in the United States, the taxpayer only may include the costs to produce or acquire the specific U.S. Component, and must exclude any other direct materials or direct labor costs related to the Manufactured Product.
Further, installation and other project-site costs (including direct costs and labor costs of incorporating the Project Components into a project) are excluded.
Statutory Percentages
For PTC and ITC projects beginning construction before 2025, the statutory percentage is 20 percent for offshore wind facilities and 40 percent for all other projects.
For PTC projects that begin construction in 2025, the percentage is 45 percent (27.5 percent for offshore wind), increasing to 50 percent in 2026 (35 percent for offshore wind) and 55 percent in 2027 and thereafter (45 percent for offshore wind in 2027 and 55 percent thereafter).
For ITC projects, the statutory percentage remains 40 percent (20 percent for offshore wind), although a recent report from the Joint Committee on Taxation states that this was not Congress’s intent and that a technical correction may be necessary to conform the statutory percentage increases for the ITC to that of the PTC.[11]
Retrofitted Projects
Consistent with long-standing guidance, the Notice allows a project to qualify as originally placed in service even if it contains some used property, as long as the fair market value of the used property is no more than 20 percent of the total value of the project (the “80/20 Rule”). This calculation is made by adding the cost of the new property to the value of the used property. The cost of the new property includes all costs properly included in the depreciable basis of the new property.
If a project meets the 80/20 Rule and is placed in service after 2022, the project is eligible for the Domestic Content Bonus Credit as long as the new property in the project meets the Domestic Content Requirement and the taxpayer otherwise complies with the requirements in the Notice.
Certification and Substantiation
The Notice also provides that a “taxpayer” reporting a Domestic Content Bonus Credit must provide a statement to the IRS certifying, as of the date the project is placed in service, that the project satisfies the Steel or Iron Requirement and the Manufactured Products Requirement and provides details concerning both the contents of the certification and its submission. In addition, the Notice makes clear that taxpayers claiming the Domestic Content Bonus Credit must maintain records substantiating compliance with the applicable requirements.
Observations
Substantiating U.S. Component costs may be challenging as component manufacturers may be unwilling to disclose such pricing information or their own margins, and even where third-party manufacturers are willing to disclose this type of information, it is unclear what documentation or evidence, if any, is needed to substantiate a third-party manufacturer’s determination of its costs.
Moreover, for those Project Components not described in the safe harbor in Table 2 of the Notice (which has been reproduced above), taxpayers likely will face uncertainty as to whether the more exacting Steel or Iron Requirement or the less exacting Manufactured Products Requirement should apply to those individual Project Components. For example, the Notice provides that the Steel or Iron Requirement applies to materials that are “structural in function” and are made “primarily of steel or iron” but fails to provide rules for determining whether a component is “primarily” made of steel or iron and does not provide a precise definition for what constitutes a construction material that is “structural in function.” Similarly, although the Notice makes clear that mere assembly does not constitute manufacturing, the Notice provides limited practical guidance on how to draw the distinction between manufacturing and assembly—a crucial distinction both for purposes of determining whether a component constitutes a Manufactured Product and for purposes of determining whether Project Components are U.S. Manufactured Products.
The Notice provides that a “taxpayer” reporting a Domestic Content Bonus Credit must make the required certification on IRS Form 8835 (Renewable Electricity Product Credit) or IRS Form 3468 (Investment Credit), or other applicable form, but does not indicate, in the case of a credit transfer under section 6418, which taxpayer must make the certification. Instructions for taxpayers with 2023 short years provide (here) that only transferors of credits need to file these source credit forms, but the Notice does not provide this level of guidance.
While the ITC is calculated on a property-by-property basis, the Domestic Content Requirement is determined on an “energy project” basis, which is defined as “a project consisting of one or more energy properties that are part of a single project.” The IRS and Treasury have not yet provided any guidance regarding what constitutes a “single project” for purposes of this definition; however, the statutory language tracks certain language in Notice 2018-59 (concerning commencement of construction), and it would be helpful if the IRS were to confirm that the “single project” definition in Notice 2018-59 applies for these purposes.
Moreover, both the Notice and Notice 2023-29 (concerning energy community bonus credits, discussed in an earlier Gibson Dunn alert here) observe that bonus credits are available with respect to “qualified property for which a valid irrevocable election under section 48(a)(5) has been made to treat such qualified property as energy property under section 48” (i.e., the ITC in lieu of PTC election). However, neither notice mentions the availability of bonus credits with respect to a specified clean hydrogen production facility for which an ITC is irrevocably elected under section 48(a)(15). While bonus credits are not available if the clean hydrogen PTC is elected under 45V (or the carbon capture and sequestration credit under section 45Q is claimed with respect to the facility), the bonus credits are apparently available if the ITC is elected for such facility. It would be helpful for the IRS and Treasury to confirm that bonus credits are available for taxpayers that elect the ITC for projects under section 48(a)(15) in the same manner as taxpayers that elect the ITC for projects under section 48(a)(5).
Effective Date
The IRS and Treasury expect to issue proposed regulations addressing the Domestic Content Requirement that would apply to taxable years ending after May 12, 2023. The Notice provides that taxpayers may rely on the rules provided in the Notice with respect to projects on which construction begins before the date that is ninety days after the date of publication of those forthcoming proposed regulations.
____________________________
[1] Unless indicated otherwise, all section references are to the Internal Revenue Code of 1986, as amended (the “Code”), and all “Treas. Reg. §” references are to the Treasury Regulations promulgated under the Code.
[2] As was the case with the so-called Tax Cuts and Jobs Act, the Senate’s reconciliation rules prevented Senators from changing the formal name of the Act. Thus, the formal name of the Inflation Reduction Act is “An Act to provide for reconciliation pursuant to title II of S. Con. Res. 14.”
[3] For purposes of this Notice, the United States includes the States, the District of Columbia, the Commonwealth of Puerto Rico, Guam, American Samoa, the U.S. Virgin Islands, and the Commonwealth of Northern Mariana Islands.
[4] Tax-exempt entities for these purposes include any organizations exempt from tax imposed by subtitle A of the Code, state and local governments, the Tennessee Valley Authority, Indian tribal governments, any Alaska Native Corporation, and rural electric cooperatives.
[5] The IRA authorizes the IRS to provide exceptions to the direct pay phaseout if (i) the inclusion of steel, iron, or manufactured products that are produced in the United States either increases the overall costs of construction of projects by more than 25 percent or (ii) there are either insufficient materials of these types produced in the United States or the materials produced in the United States are not of satisfactory quality.
[6] In the case of projects subject to prevailing wage and apprenticeship requirements, failure to satisfy those requirements reduces the bonus credits amount to 2 percent (for PTC projects) or 2 percentage points (for ITC projects).
[7] For purposes of this Notice, a “manufacturing process” is the application of processes to alter the form or function of materials or of elements of a product in a manner adding value and transforming those materials or elements so that they represent a new item functionally different from the functionality that would result from mere assembly of the elements or materials.
[8] The Steel or Iron Requirement applies in a manner consistent with Section 661.5(b) and (c) of title 49 of the Code of Federal Regulations (the “CFR”). 49 CFR §§ 661.1 through 661.21 (also known as the “Buy America” requirements).
[9] The Manufactured Products Requirement applies in a manner consistent with 49 CFR § 661.5(d).
[10] Direct costs are defined by reference to Treas. Reg. § 1.263A-1(e)(2)(i).
[11] Joint Committee on Tax’n, Description of Energy Tax Law Changes Made by Public Law 117-169, JCX 5-23 (April 17, 2023), at n. 201.
This alert was prepared by Josiah Bethards, Emily Brooks, Mike Cannon, Matt Donnelly, Alissa Fromkin Freltz*, Duncan Hamilton, Kathryn Kelly, and Simon Moskovitz.
Gibson Dunn lawyers are available to assist in addressing any questions you may have about these developments. To learn more about these issues, please contact the Gibson Dunn lawyer with whom you usually work, any member of the firm’s Tax or Power and Renewables practice groups, or the following authors:
Tax Group:
Michael Q. Cannon – Dallas (+1 214-698-3232, mcannon@gibsondunn.com)
Matt Donnelly – Washington, D.C. (+1 202-887-3567, mjdonnelly@gibsondunn.com)
Kathryn A. Kelly – New York (+1 212-351-3876, kkelly@gibsondunn.com)
Josiah Bethards – Dallas (+1 214-698-3354, jbethards@gibsondunn.com)
Emily Risher Brooks – Dallas (+1 214-698-3104, ebrooks@gibsondunn.com)
Duncan Hamilton– Dallas (+1 214-698-3135, dhamilton@gibsondunn.com)
Simon Moskovitz – Washington, D.C. (+1 202-777-9532 , smoskovitz@gibsondunn.com)
Power and Renewables Group:
Gerald P. Farano – Denver (+1 303-298-5732, jfarano@gibsondunn.com)
Peter J. Hanlon – New York (+1 212-351-2425, phanlon@gibsondunn.com)
Nicholas H. Politan, Jr. – New York (+1 212-351-2616, npolitan@gibsondunn.com)
*Alissa Fromkin Freltz is an associate working in the firm’s Washington, D.C. office who currently is admitted to practice only in Illinois and New York.
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
What Comes Next? Looking Forward By Looking Back
On April 28, 2023:
- The Board of Governors of the Federal Reserve System (“Federal Reserve”) released the results of its review of the supervision and regulation of Silicon Valley Bank (“SVB”);
- The Federal Deposit Insurance Corporation (“FDIC”) released its report detailing an internal review of the agency’s supervision of Signature Bank (“Signature”);
- The New York State Department of Financial Services released its review of its supervision and closure of Signature; and
- The Government Accountability Office released its preliminary review of the federal banking agencies’ actions related to the failures of SVB and Signature.
The reports in part assign, and in part accept, blame for the failures of SVB and Signature to the institutions’ boards of directors and management and the agencies’ own missteps in their oversight of the institutions through their supervisory and regulatory authorities. The Federal Reserve’s report is also critical of its own tailoring approach in response to the 2018 Economic Growth, Regulatory Relief, and Consumer Protection Act (“EGRRCPA”).
Rather than summarize the reports’ details of the events leading up to the failures of SVB and Signature, which have been extensively covered, we examine the federal banking agencies’ expected collective response to the recent failures of SVB, Signature, and First Republic Bank, self-liquidation of Silvergate Bank, resulting financial distress across the financial markets broadly, and volatility experienced by similarly sized regional banks acutely. We also examine relevant considerations for FinTechs or other financial services or technology companies that partner with banks for the delivery of innovative financial products and services.
The expected response will shape and shift the regulatory landscape going forward for institutions of all sizes and their partners, and could result in significant changes to the regulatory and supervisory oversight of those institutions and related supervisory expectations and processes. In that regard, there are two takeaways from the reports:
- We can look forward to the expected regulatory response by looking back at the fundamental risk management principles codified in the Dodd-Frank Act and the changes made to the alignment of those principles under EGRRCPA. The more immediate impact will be felt through the supervisory process and quickly evolving supervisory expectations because proposed rulemakings could take “several years” to effect (as Vice Chair for Supervision Barr acknowledges in his cover letter).
- All relevant stakeholders should be actively engaged in the rulemaking process, both to facilitate a thoughtful approach to proposed regulation that weighs the costs and benefits of proposed actions, and to help design an adjusted and balanced framework that promotes safety and soundness and resolvability, provides clarity, reduces complexity and, equally as important, does not diminish banks’ critical role as financial intermediaries or create unintended harmful consequences to the broader economy.
I. Background: Dodd-Frank and EGRRCPA
The Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 (“Dodd-Frank Act”) established enhanced prudential standards for bank holding companies and foreign banking organizations with total consolidated assets of $50 billion or more and nonbank financial companies designated by the Financial Stability Oversight Council for supervision by the Federal Reserve. Under the Dodd-Frank Act, those enhanced prudential standards include enhanced risk-based and leverage capital, liquidity, risk management and risk committee requirements, a requirement to submit a resolution plan, single-counterparty credit limits, supervisory and company-run stress testing requirements, and other prudential standards that the Federal Reserve determines are appropriate.
However, EGRRCPA subsequently raised the minimum asset threshold for application of enhanced prudential standards from $50 billion to $250 billion in total consolidated assets, while (i) providing the Federal Reserve discretion in determining whether an institution with assets of $100 billion or more must be subject to such standards and (ii) enabling a more “tiered” and “tailored” enhanced prudential standards regime for large banks. In 2019, the Federal Reserve issued a final rule establishing four categories for determining the applicability and stringency of prudential standards:
- Category I (U.S. global systemically important bank holding companies (“U.S. G-SIBs”));
- Category II (banking organizations with $700 billion or more in total consolidated assets or $75 billion or more in cross-jurisdictional activity);
- Category III (banking organizations with $250 billion or more in total consolidated assets or $75 billion or more in weighted short-term wholesale funding, nonbank assets, or off-balance sheet exposure and that do not meet the criteria for Category I or II); and
- Category IV (banking organizations with at least $100 billion in total consolidated assets and that do not meet the criteria for Category I, II, or III).
The Federal Reserve’s visual depicting the current framework is available here.
II. A Return to Post-Dodd-Frank Act Principles of Oversight and Supervision (or More)?
Vice Chair for Supervision Barr’s cover letter, the Federal Reserve’s report, and the other agencies’ reports forecast expected adjustments to the regulatory framework to align it more closely with the fundamental risk management principles codified in the Dodd-Frank Act, to the extent not limited by or inconsistent with legislative changes made under EGRRCPA. The expected response echoes statements made by Vice Chair for Supervision Barr and other federal banking agency leaders in speeches and other settings, including Congressional testimony, some of which pre-date the most recent bank failures and disruptions in the markets and broader economy. The federal bank regulatory agencies under the Biden administration have signaled for some time their desire to re-align Dodd-Frank Act risk management principles of oversight and supervision—at least to the extent not limited by changes made under EGRRCPA—and the agencies’ reports reaffirm that. Large banks (i.e., banks with total assets of $100 billion or more) should expect an acceleration in the number and scope of proposals that modify the regulatory framework, including proposals that push down certain elements currently applicable only to U.S. G-SIBs.
Vice Chair for Supervision Barr’s cover letter to the Federal Reserve’s report highlights expected regulatory initiatives that are clearly put forth and of likely immediacy for institutions with $100 billion or more in total consolidated assets: capital, liquidity, resolvability, and stress testing. It will be important that any proposals not simply be reflexive but, instead, be thoughtfully designed, provide clarity, assess the costs and benefits, and minimize potential downside to the broader economy.
- Capital. Vice Chair for Supervision Barr states in his cover letter to the report “this experience has emphasized why strong bank capital matters,” highlights the need “to bolster resiliency” and confirms the Federal Reserve is “going to evaluate how to improve [its] capital requirements in light of lessons learned from SVB.” He then adds that “[s]ome steps already in progress include the holistic review of our capital framework [and] implementation of the Basel III endgame rules.” These echo prior statements and remarks for the need to strengthen capital requirements by Barr, including as far back as his nomination hearing before the Senate Banking Committee, as well as similar statements made by Acting Comptroller Hsu and FDIC Chairman Gruenberg in different contexts. As Barr has previously noted, large institutions should expect those enhanced regulatory capital requirements to align with the final set of Basel III standards aimed at “further strengthen[ing] capital rules by reducing reliance on internal bank models and better reflect risks from a bank’s trading book and operational risks”[1] and any proposal should be expected to follow shortly. Barr’s cover letter also suggests the Federal Reserve “should require a broader set of firms to take into account unrealized gains or losses on available-for-sale securities, so that a firm’s capital requirements are better aligned with its financial positions and risk.” As with prior rulemakings, any proposal, if and when finalized, would be implemented with appropriate phase-in periods and likely would take “several years” to take effect, as noted by Barr himself.
- Liquidity. Vice Chair for Supervision Barr’s cover letter indicates the Federal Reserve is “also going to evaluate how [the Federal Reserve] supervise[s] and regulate[s] liquidity risk, starting with the risks of uninsured deposits,” adding that “liquidity requirements and models should better capture the liquidity risk of a firm’s uninsured deposit base” and the Federal Reserve “should re-evaluate the stability of uninsured deposits and the treatment of held to maturity securities in … standardized liquidity rules and in a firm’s internal liquidity stress tests.” He then adds the Federal Reserve “should … consider applying standardized liquidity requirements to a broader set of firms.” He concludes that “[a]ny adjustments to [the] liquidity rules would … have appropriate transition rules, and thus would not be effective for several years.”
- Resolvability. Vice Chair for Supervision Barr’s cover letter also indicates that, following on the October 14, 2022 Advance Notice of Proposed Rulemaking (“ANPR”) issued by the Federal Reserve and FDIC, the federal banking agencies will plan to propose a long-term debt requirement for large banks that are not U.S. G-SIBs. The earlier ANPR was issued to explore whether and how to strengthen resolution-related standards applicable to large banking organizations (i.e., Category II and Category III banking organizations under the tailoring rules). The ANPR considered whether large banking organizations should be subject to resolution requirements similar to those required of U.S. G-SIBs, including total loss-absorbing capacity, long-term debt, clean-holding company requirements, and related requirements.
- Stress Testing. Vice Chair for Supervision Barr’s cover letter includes in the list of steps already in progress “the use of multiple scenarios in stress testing” and notes the Federal Reserve will be “revisiting” the “coverage and timeliness” (i.e., applicable transition periods) of stress tests for some firms.
III. A Shift in Supervisory Expectations and Processes
Changes to the regulatory framework will take a number of years to effect, taking into account sometimes lengthy notice and public comment periods and the implementation of final rules and phase-in periods accompanying their implementation. As a result, and as a natural response to criticisms leveled (by regulators) at the oversight and supervision (by regulators) of SVB and Signature, banks of all sizes should anticipate a noticeable and swift shift in supervisory expectations and the communication and enforcement of those expectations.
The reports signal several areas of concern that will (if not already) be areas of heightened supervisory focus that, if not properly managed from a risk perspective, could lead more quickly to ratings downgrades, formal or informal enforcement actions, or other supervisory actions. Such areas of focus include: governance and risk management functions, including internal audit; management challenge and accountability; liquidity risk management; interest rate risk management; reliance on uninsured deposits and concentrations in the deposit base; and rapid growth, concentrated business models, or novel activities (e.g., FinTech or crypto), regardless of asset size. Other areas of focus or expected change for immediate consideration include:
- Developing a “culture that empowers supervisors to act in the face of uncertainty” and improves the “speed, force, and agility of supervision.” Vice Chair for Supervision Barr’s cover letter states this directly and the report highlights this in several instances, and the intent is clear: supervisory staff should be empowered to escalate issues and act more quickly and decisively—and not simply through the issuance of more matters requiring attention (“MRAs”) or matters requiring immediate attention (“MRIAs”). This could include empowering supervisory staff to escalate matters and move more quickly to downgrade component or composite ratings or to issue formal or informal enforcement actions or take other actions without the need for “consensus around supervisory judgments.” Institutions with MRAs or MRIAs that have remained open for a protracted period and where expected remediation dates have been extended should expect supervisory staff to act more quickly and decisively, including escalation to the level of enforcement actions, in the absence of meaningful progress or remediation. More frequent targeted exams should also be expected by institutions with open MRAs, MRIAs, or other unresolved findings.
- Ratings downgrades and formal or informal enforcement actions may have a number of significant collateral consequences to banks and their holding companies and non-bank affiliates, including the ability to engage in financial activities under Section 4(k) of the Bank Holding Company Act, potential increases to deposit insurance assessments, eligibility for primary credit at the Discount Window, and the ability to expand through mergers and acquisitions, including interstate acquisitions or branching.
- In addition, Vice Chair for Supervision Barr’s cover letter notes “the Federal Reserve generally does not require additional capital or liquidity beyond regulatory requirements for a firm with inadequate capital planning, liquidity risk management, or governance and controls. We need to change that in appropriate cases. … [L]imits on capital distributions or incentive compensation could be appropriate and effective in some cases.” Institutions should begin to assess and better understand these various collateral consequences as part of their routine examination preparation processes.
- Consequences and impacts of a U.S. debt default. A U.S. debt default would be unprecedented and the macroeconomic effects of such a default are uncertain, but institutions should be preparing for such a scenario, including a prolonged default, and be ready to activate contingency plans if negotiations stall or deteriorate. There are several issues that immediately come to mind and, although there is no precedent, prior discussions included in the minutes of Federal Open Market Committee (“FOMC”) meetings from August 2011 and October 2013 should inform current expectations.
- First, in August 2011 and October 2013, the FOMC suggested that Federal Reserve operations should treat defaulted Treasury securities or Treasury securities with delayed payments in the same manner as non-defaulted securities in open market operations and at the Discount Window, but with defaulted securities valued at their own potentially reduced market prices.
- Compare that with the recently announced Bank Term Funding Program, under which collateral valuation is 100% of par value regardless of the current market value of the collateral. For Discount Window borrowings, collateral is traditionally valued at a fair market value estimate; however, as of March 15, 2023, the Federal Reserve Banks have been lending at par value for collateral that is eligible for the Bank Term Funding Program, including Treasury securities, agency debt, and agency mortgage-backed securities.[2]
- Relatedly, institutions are reminded to test their Discount Window and Federal Home Loan Bank borrowing capacity and ensure that all collateral and related documentation are in order and technical processes in place (and tested) to ensure immediate and timely access to those contingent sources of funding. The Bank Term Funding Program also remains open and available to financial institutions that already have Discount Window borrowing documentation under the Federal Reserve Banks’ Operating Circular No. 10 (Lending). Although the Dodd-Frank Act requires the Federal Reserve to publish information on individual discount window borrowers and transactions, that information is published on a two-year lag.
- Second, in August 2011 and October 2013, the federal financial regulatory agencies were prepared to issue interagency guidance covering certain regulatory and supervisory issues—which we expect could be refreshed in the coming weeks. The 2011 and 2013 draft interagency guidance intended to clarify that:
- There would be no change in the risk-based capital treatment (i.e., no change in risk weighting) of Treasury securities or other securities issued or guaranteed by the U.S. government or its agencies, as well as U.S. government-sponsored enterprises, for which a payment had been missed. Examiners would not adversely classify or criticize those securities, and their treatment under other regulations (e.g., Regulation W) would be unaffected.
- Institutions that experience balance sheet growth from unusually large deposit inflows driven primarily by money market funds moving out of Treasury securities into cash or holding additional cash as contingency, or draws on existing lines of credit, which could result in a temporary decline in regulatory capital ratios, were encouraged to contact their regulators to address the impacts to regulatory capital ratios. In 2023, the federal banking agencies should be expected to provide some relief around the supplementary leverage ratio and Tier 1 leverage ratio should institutions be at risk of breaching prompt corrective action.
- Third, in August 2011 and October 2013, the Department of the Treasury was planning to prioritize interest and principal payments, which, if implemented in 2023, could eliminate the need to plan for scenarios in which defaulted securities are trading in the market.
- First, in August 2011 and October 2013, the FOMC suggested that Federal Reserve operations should treat defaulted Treasury securities or Treasury securities with delayed payments in the same manner as non-defaulted securities in open market operations and at the Discount Window, but with defaulted securities valued at their own potentially reduced market prices.
- Transition periods and “pulling forward” forward large bank standards by applying them to smaller banks. Institutions that are growing in size and transitioning supervisory categories tied to relevant asset thresholds (e.g., $100 billion or $250 billion in total consolidated assets) should be prepared to adhere to the enhanced prudential standards of the next supervisory category, including on a pro forma An inability to demonstrate adherence to the next supervisory category’s enhanced prudential standards could slow growth, either through prolonged merger application review and approval timelines or regulators throttling growth through other means. All institutions, regardless of size, should also expect regulators to carefully examine transition periods both for existing rules as well as proposed rules such that enhanced prudential standards, and required compliance therewith, could apply more quickly to any institution that transitions from one supervisory category to the next.
- Credit risk and commercial real estate (“CRE”) loans. Commercial credit risk has been cited by the agencies as an area of supervisory focus beginning as early as the OCC’s Spring 2021 Semiannual Risk Perspective and was most recently cited in the Federal Reserve’s May 2023 Financial Stability Report survey of risks to financial stability. Institutions with concentrations in CRE loans should expect continued heightened scrutiny of their CRE portfolios, with a focus on risk management and capital levels. Institutions are reminded of the CRE interagency guidance from December 6, 2006, “Concentrations in CRE Lending, Sound Risk Management Practices,” and the October 3, 2009 “Policy Statement on Prudential Commercial Real Estate (CRE) Loan Accommodations and Workouts,” on which the federal banking agencies invited comment in August and September 2022.
- Incentive compensation. Vice Chair for Supervision Barr’s cover letter notes that regulators “should consider setting tougher minimum standards for incentive compensation programs and ensure banks comply with the standards [regulators] already have.” The report highlights the various interagency guidance on executive compensation practices but only briefly notes that the Federal Reserve and five other federal financial regulatory agencies have not yet issued a final rule implementing Section 956 of the Dodd-Frank Act, which requires the regulators to issue rules prohibiting types and features of incentive compensation arrangements that encourage inappropriate risk-taking at covered financial institutions (i) by providing excessive compensation, fees, or benefits or (ii) that could lead to material financial loss. Although not highlighted by Barr in his cover letter as an initiative already in progress, incentive compensation arrangements may become an area of interest through the supervisory process and future proposed rulemakings remain a possibility.
- Operational resilience and cybersecurity. Though unrelated to the reports and the expected response (and certainly never suggested as a root cause of any failure), cybersecurity remains a point that is always worthy of highlighting because the potential impact to operational risk from cybersecurity threats remains a supervisory focus in an increasingly digital world and an environment where cybersecurity risks are ever-increasing. Cybersecurity will always remain an area of supervisory focus and institutions should be mindful that any failures to address supervisory concerns related to cybersecurity may result more quickly in formal or informal supervisory responses.
IV. Implications for Bank Partners
FinTechs that partner with banks to deliver regulated financial services should expect additional scrutiny from both their bank partners and their bank partners’ regulators. Although these partnerships can take different forms—some with FinTechs positioned as clients of the bank and others with FinTechs acting as a program manager (i.e., third-party service provider) to the bank—FinTechs should be prepared for enhanced due diligence and, as importantly, potential disruptions.
- Accounting for increasingly agile regulators. As federal or state supervisory functions are empowered to move more quickly to ratings downgrades or formal or informal enforcement or other actions to enforce supervisory expectations, any actions could have adverse effects on those FinTechs, which may create disruptions in the delivery of services to end-users. This dependence on bank partnerships reinforces the need for FinTechs to develop robust business continuity plans that provide for necessary diversification of bank partners and, in the course of negotiating such relationships, ensure sufficient contractual flexibility exists to adopt necessary redundancies. In addition, FinTechs must also remain cognizant of the federal and state bank regulatory agencies’ authority to examine and regulate bank service providers, which may give rise to regulatory criticism more tailored to the FinTech relationship.
- Clear disclosure of bank services. To the extent that FinTechs are marketing products and services enabled by banks, regulators are more apt to scrutinize terms of service, marketing materials, and related disclosures to assess the allocation of roles and responsibilities between the bank and the FinTech. For those products that potentially implicate FDIC insurance, the FDIC will review for compliance with the FDIC’s 2022 final rule regarding advertising or other representations about FDIC deposit insurance (12 C.F.R. Part 328, Subpart B). To ensure compliance, banks and FinTechs should, at a minimum, ensure subject materials: (a) clearly disclose that the FinTech offering the service is not an insured bank; (b) identify the insured bank(s) where any customer funds may be held on deposit; and (c) communicate that non-deposit products are not FDIC-insured products and may lose value.
- Qualifications for FDIC “pass-through” deposit insurance. FinTechs that partner with banks also should refresh on FDIC regulations (12 C.F.R. §§ 330.5 and 330.7) and related guidance for “pass-through” deposit insurance, including recordkeeping and other requirements, to ensure compliance therewith. This should include re-examining program agreements with bank partners to ensure the proper mechanics are in place to enable the respective parties to comply with those requirements. Requirements for pass-through deposit insurance coverage include:
- Funds must be owned by the principal and not the third party who established the deposit account and placed the funds (i.e., the fiduciary, custodian, or agent who is placing the funds);
- The bank’s account records must indicate the agency nature of the account;
- The records of the bank, the fiduciary, custodian, or agent, or a third party must indicate both the principals’ identities as well as their ownership interest in the deposit; and
- Deposit terms (i.e., the interest rate and maturity date) for accounts opened at the bank must match the terms the third-party agent offers the customer (if the terms do not match, the fiduciary, agent, or custodian might be deemed to be the legal owner of the funds by the FDIC; a fiduciary, custodian, or agent may retain a portion of the interest (as the third party’s fee) without precluding pass-through deposit insurance coverage).
Like banks, their partners find themselves navigating an increasingly complex regulatory environment. While the regulatory expectations are not new, the renewed focus of banking regulators requires both agility and vigilance of all concerned.
V. Conclusions
A key takeaway not expressly cited in the reports is that perceived complacency in upholding risk management obligations will result in the regulatory framework reverting to and aligning more closely with the fundamental risk management principles codified in the Dodd-Frank Act, to the extent not limited by or inconsistent with legislative changes made under EGRRCPA. Large banks should expect a number of proposed rulemakings to follow these events, as laid out in Vice Chair for Supervision Barr’s cover letter to the Federal Reserve’s report. Notwithstanding regulators’ desire to move quickly, because of the amount of time to effect proposed rule changes and to implement final rules, together with any applicable phase-in periods, large banks should anticipate certain proposals may not be effective for several years. On the other hand, banks of all sizes should expect that additional oversight and supervision will ratchet up quickly, with increased scrutiny on boards of directors’ and management’s ability to safely and soundly risk manage their organizations consistent with the fundamental risk management principles codified in the Dodd-Frank Act. Institutions of all sizes should be similarly prepared that any failure, or even perceived failure, to satisfy supervisory expectations may lead more quickly to formal or informal enforcement actions, ratings downgrades, or other consequences to the organization.
FinTechs that partner with banks for the delivery of innovative financial products and services should expect additional scrutiny from both their bank partners and from relevant regulators. Moreover, if regulators are empowered to move more quickly to ratings downgrades or formal or informal enforcement or other actions to reinforce supervisory expectations, these actions could adversely impact those partners and/or their ability to offer products and services with their existing bank partners.
__________________________
[1] Michael S. Barr, “Why Bank Capital Matters” (speech at the American Enterprise Institute, Washington D.C., Dec. 1, 2022, available at: https://www.federalreserve.gov/newsevents/speech/barr20221201a.htm).
[2] See “Collateral Valuation,” available at: https://www.frbdiscountwindow.org/Pages/Collateral/collateral_valuation.
Gibson Dunn’s Distressed Banks Resource Center provides resources and regular updates to our clients. Please check the Resource Center for the latest developments.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. Please feel free to contact the Gibson Dunn lawyer with whom you usually work, any member of Gibson Dunn’s Global Financial Regulatory, Financial Institutions or FinTech and Digital Assets practice groups, or the firm’s *** Distressed Bank Working Group, or the following authors:
Jason J. Cabral – New York (+1 212-351-6267, jcabral@gibsondunn.com)
Sara K. Weed – Washington, D.C. (+1 202-955-8507, sweed@gibsondunn.com)
Ed Batts – Palo Alto (+1 650-849-5392, ebatts@gibsondunn.com)
Jin Hee Kim – New York (+1 212-351-5371, jhkim@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
In a May 11, 2023 webcast, the Department of Commerce’s CHIPS Program Office (“CPO”) provided a deep dive on the Environmental Questionnaire required for funding applications and pre-applications. This questionnaire is designed specifically to assist the CPO in “determining the appropriate level of environmental review required under the National Environmental Policy Act (“NEPA”) and related laws.”[1] We have provided a fulsome discussion of CHIPS Act funding in our previous alerts [here], [here], and [here].
The key takeaway from this most recent discussion is that the Environmental Division of the CPO intends to work closely with applicants to facilitate their development of necessary environmental data and to ensure applicants are prepared for permitting processes and review under NEPA. The CPO stressed, however, that it intends to prioritize applications “that demonstrate a clear path” to satisfying these requirements in a timely manner.[2]
This guidance applies only to applicants applying for funding pursuant to the CHIPS Act’s first Notice of Funding Opportunities (“NOFO”), which focuses on commercial fabrication facilities.[3] Detailed instructions for other applicants will be released after the publication of the second and third NOFOs later in 2023.
I. The Environmental Questionnaire
All applicants for CHIPS Funding must submit responses to the Environmental Questionnaire, both at the pre-application and final application stages. Applicants seeking funding for multiple projects need only submit one Environmental Questionnaire for their entire application, although the CPO stressed that multiple project-specific Environmental Questionnaires are welcome.[4]
During the May 11th webcast, CPO Environmental Division staff described this questionnaire as serving a number of purposes:[5]
- Allowing the CPO to assess the environmental risks and merits of every project;
- Helping the Department of Commerce fulfill its statutory and regulatory duties under NEPA and other environmental laws;
- Supplementing and providing additional context to the “Climate and Environmental Responsibility Plan” that each applicant must submit pursuant to Section IV.G.11 of the NOFO;[6]
- Helping applicants better understand the environmental impacts of their projects and determine how to mitigate these impacts; and
- Helping applicants determine their legal obligations under NEPA and other environmental laws.
The Environmental Questionnaire consists of 26 questions related to the following topics:[7]
|
A. Project Description |
B. Project Site & Affected Environment |
C. Resource Consumption Rates & Effluent Emissions Streams and Impacts |
|
D. Tribal, Historic & Cultural Resources |
E. Project Setting |
F. Vegetation & Wildlife Resources |
|
G. Conservation Areas |
H. Coastal Zone & Navigable Waters |
I. Wetlands |
|
J. Floodplains |
K. Endangered Species |
L. Land Use & Zoning |
|
M. Solid Waste Management |
N. Hazardous or Toxic Substances |
O. Impacts to Water Quality & Water Resources |
|
P. Water Supply & Distribution System |
Q. Wastewater Collection & Treatment Facilities |
R. Environmental Justice & Socioeconomics |
|
S. Transportation (Streets, Traffic & Parking) |
T. Air Quality |
U. Greenhouse Gases & their Environmental Effects |
|
V. Noise |
W. Health & Safety |
X. Permits & Other Government Agency Involvement |
|
Y. Public Notification & Controversy |
Z. Environmental Experience & Approach |
For each topic, applicants are required to provide “a sufficient level of documentation and analysis to inform CPO’s assessment of the appropriate level of NEPA review” and are encouraged to attach any relevant documents, such as permit applications, background research, field investigations and surveys, and any past planning or studies.[8]
II. Evaluation Criteria for the Environmental Questionnaire
As discussed in our previous alert, the CPO will evaluate all applications and pre-applications according to six key criteria: (1) economic and national security objectives; (2) commercial viability; (3) financial strength; (4) technical feasibility and readiness; (5) workforce development; and (6) projects’ broader impacts.[9] During the May 11th webcast, CPO staff emphasized that assessment of an applicant’s Environment Questionnaire will focus primarily on technical feasibility and readiness.[10]
The commercial fabrication facilities funded under the first NOFO are all likely to be subject to a wide array of federal, state, and local environmental and permitting requirements. Applicants must assure the CPO that funded projects will be able to satisfy these legal requirements. To demonstrate feasibility, applicants must clearly identify the necessary environmental compliance and permitting steps for each proposed project and, if needed, for individual activities within each project.[11]
Applicants should not be deterred, however, from beginning the application process simply because they do not yet have all necessary environmental information. The CPO staff repeatedly emphasized that, while more information is always better, applicants need only provide as much detail as is currently available to them.[12] However, rather than simply skipping inapplicable or not-yet-knowable questions, applicants should provide a brief statement explaining why the question is not applicable or what information is needed to provide a full answer.[13]
a. Feasibility of NEPA Review
NEPA sets forth “a national policy that encourages productive and enjoyable harmony between man and his environment” and therefore requires federal agencies to consider the environmental impacts of all major federal actions that significantly affect the quality of the human environment.[14] These “major federal actions” include “projects and programs entirely or partly financed” by a federal agency, including the Department of Commerce.[15]
The Department of Commerce is therefore legally obligated under NEPA to assess the environmental impact of any project funded under the CHIPS Program. This involves a dynamic review process that will vary based on the project’s potential environmental impacts:
- Projects initially assessed as likely to cause significant environmental impacts will require a fulsome Environmental Impact Statement (“EIS”).[16] The drafting of an EIS is subject to public notice and comment, including comments from impacted communities and the Environmental Protection Agency. Once the EIS is finalized, the National Institute of Standards and Technology (“NIST”) will issue a Record of Decision, indicating whether the project will be allowed to proceed.[17]
- Projects initially assessed as unlikely to cause significant environmental impacts require a brief, publicly accessible Environmental Assessment (“EA”).[18] If the EA demonstrates that the proposed project will not have significant environmental effects, the NIST will issue a Finding of No Significant Impact.[19] If, however, the EA suggests that significant environmental effects may occur, the project will require an EIS, described above.
The Environmental Questionnaire is designed to help the CPO determine which level of NEPA review any given project will require. This determination informs not just the substantive environmental data an applicant may be called to produce, but also the timeline for completion of the review. If an applicant’s project is likely to cause significant environmental impacts, for example, that applicant will be subject to a long notice-and-comment period and will be required to produce detailed data for an EIS.
Notably, the CPO has emphasized that applicants should be prepared to produce additional environmental data and documentation for this NEPA review. While the Environmental Questionnaire helps the CPO assess a project’s likely level of NEPA review, an EA or EIS may demand additional information. This may include detailed descriptions of site-specific impacts, descriptions of the purpose or need for a proposed project, a discussion of reasonable alternatives, and more.[20]
The CPO has published a detailed overview of the NEPA review process online.[21]
b. Permitting Considerations
The Environmental Questionnaire’s Question X specifically discusses permitting issues and provides applicants a useful model to assess the status of their permitting needs.
Applicants should be prepared to identify any federal, tribal, state, or local environmental plans or reviews that will be needed for each proposed project. These may include, for example, Clean Water Act 404 permits, stormwater management plans, coastal zone management and shoreline management plans, and Clean Air Act permits.[22] If available, copies of these permits or permit applications should be attached to the Environmental Questionnaire. In its May 11th webcast, the CPO recommended laying out all required permits in a simple table, such as the following:[23]
The CPO staff emphasized that applicants needn’t have all permits secured at the time of submitting their pre-application or even full application. However, a clear understanding of a project’s permitting timeline and requirements would allow the Environmental Division to encourage prompt review of permit applications by coordinating with other federal and state agencies as necessary.[24] Moreover, if an applicant identifies additional permitting requirements after submitting a pre-application, the CPO encourages the applicant to reach out to the Environmental Division immediately, rather than waiting to raise these concerns in a later final application.[25]
III. Role of the CPO Environmental Division
Applicants for CHIPS Act funding will interface directly with the Environmental Division of the CPO when addressing the environmental impacts of proposed projects.
The Environmental Division holds dual roles within the CPO. First, it is tasked with ensuring that the Department of Commerce satisfies all applicable environmental laws and requirements connected to CHIPS-funded programs. Second, the Environmental Division collaborates with federal and state agencies, as well as with applicants, to ensure “efficient, effective, and predictable reviews that result in informed and environmentally responsible decisions.”[26] This latter mission involves working closely with applicants to identify gaps in their data that could cause problems later in the NEPA review or permitting process.
In general, applicants for CHIPS funding that have already submitted an Environmental Questionnaire are invited to request meetings with the CPO’s Environmental Division. However, in limited circumstances, the Environmental Division will meet with potential applicants prior to submission of their pre-application or full application. To qualify for such a meeting, a potential applicant must satisfy all of the following three criteria:[27]
- The potential applicant must have filed a Statement of Interest with the CPO;
- The potential applicant must be currently eligible to apply for CHIPS funding under an open NOFO; and
- The potential applicant either:
- Requires support with Clean Air Act or Clean Water Act permits; or
- Is currently drafting NEPA environmental review documents and has questions related to these documents.
IV. Resources and Templates
The CPO has prepared and published a number of resources for the preparation of applications and pre-applications under the first NOFO, including materials on environmental compliance requirements.[28]
The CPO maintains “CHIPS and Environmental Compliance“ FAQs online, which it plans to update regularly throughout the funding cycle. These FAQs primarily relate to the NEPA review process, as well as other environmental laws that may be relevant to an applicant’s project. In the May 11th webcast, the CPO indicated that it will be publishing additional FAQs on the NEPA review process soon.[29] General FAQs are also available on the CHIPS for America website.
In addition to CHIPS-specific environmental guidance, the CPO suggests that applicants refer to publications by environmental regulatory agencies when assessing permitting requirements and other legal obligations. These may include, but are not limited to, NEPA.gov, the National Marine Fisheries Service, the Advisory Council on Historic Preservation, the U.S. Army Corps of Engineers’ guidance on Section 404 of the Clean Water Act, and the U.S. Fish & Wildlife Service’s ECOS and IPaC systems.
Additional resources can be found at the CHIPS for America Guides and Templates webpage.
V. How Gibson Dunn Can Assist
Gibson Dunn has an expert team tracking implementation of the CHIPS Act closely, including semiconductor industry subject matter experts and public policy professionals. Senior members of Gibson Dunn’s Public Policy Practice Group have more than 40 years of combined experience on Capitol Hill. Our team includes former congressional staff and administration officials who have significant experience tracking, developing, and implementing legislation and regulations.
Our team is available to assist eligible clients to secure funds throughout the application process. We also can engage with our extensive contacts at the Department of Commerce and other federal agencies to facilitate dialogue with our clients and discuss the structure of future CHIPS Act programs being developed.
_________________________
[1] CHIPS for America Guide: Environmental Questionnaire (Mar. 27, 2023), https://www.nist.gov/system/files/documents/2023/03/27/Environmental-Questionnaire.pdf [hereinafter, Environmental Questionnaire].
[2] Department of Commerce Webcast (May 11, 2023).
[3] 5 U.S.C. § 4651(2); U.S. Dep’t of Commerce Nat’l Institute of Standards and Technology Notice of Funding Opportunity, CHIPS Incentives Program—Commercial Fabrication Facilities, https://www.nist.gov/system/files/documents/2023/02/28/CHIPS-Commercial_Fabrication_Facilities_NOFO_0.pdf [hereinafter, NOFO].
[4] Department of Commerce Webcast (May 11, 2023).
[5] Id.
[6] NOFO at 56. This “Climate and Environmental Responsibility Plan” must detail how a project will meet climate and environmental goals relating to: (1) energy consumption and use of renewable energy; (2) climate resilience; (3) water consumption and conservation; (4) sustainability transparency; and (5) community and environmental justice impacts. Id.
[7] Environmental Questionnaire.
[8] Id. at 1.
[9] NOFO at 58–64.
[10] Department of Commerce Webcast (May 11, 2023).
[11] See NOFO at 18–19.
[12] Department of Commerce Webcast (May 11, 2023).
[13] Id.
[14] National Environmental Policy Act, 42 U.S.C. § 4321 (1970).
[15] 40. C.F.R. § 1508.1 (2020).
[16] 40 C.F.R. § 1502 (2020).
[17] 40 C.F.R. § 1505.2 (2020).
[18] 40 C.F.R. § 1501.5 (2020).
[19] 40 C.F.R. § 1501.6 (2020).
[20] Department of Commerce Webcast (May 11, 2023).
[21] CHIPS for America: CHIPS Overview of NEPA and Environmental Reviews (last accessed May 15, 2023), https://www.nist.gov/system/files/documents/2023/04/20/3.18.23-CHIPS%20for%20America%20Overview%20of%20NEPA%20and%20Environmental%20Reviews.pdf.
[22] Environmental Questionnaire at 6.
[23] Department of Commerce Webcast (May 11, 2023).
[24] Id.
[25] Id.
[26] Id.
[27] Id.
[28] CHIPS for America Guides and Templates: CHIPS Incentives Program – Commercial Fabrication Facilities (last accessed May 15, 2023), https://www.nist.gov/chips/guides-and-templates-chips-incentives-program-commercial-fabrication-facilities; CHIPS for America: Environmental Compliance (last accessed May 15, 2023), https://www.nist.gov/chips/environmental-compliance.
[29] Department of Commerce Webcast (May 11, 2022).
The following Gibson Dunn lawyers prepared this client alert: Michael Bopp, Roscoe Jones, Jr., Ed Batts, Amanda Neely, Danny Smith, and Sean Brennan.*
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work in the firm’s Public Policy practice group, or the following authors:
Michael D. Bopp – Co-Chair, Public Policy Group, Washington, D.C. (+1 202-955-8256, mbopp@gibsondunn.com)
Roscoe Jones, Jr. – Co-Chair, Public Policy Group, Washington, D.C. (+1 202-887-3530, rjones@gibsondunn.com)
Ed Batts – Palo Alto (+1 650-849-5392, ebatts@gibsondunn.com)
Amanda H. Neely – Washington, D.C. (+1 202-777-9566, aneely@gibsondunn.com)
Daniel P. Smith* – Washington, D.C. (+1 202-777-9549, dpsmith@gibsondunn.com)
*Daniel P. Smith is of counsel working in the Washington, D.C. office who is admitted only in Illinois and practicing under supervision of members of the District of Columbia Bar under D.C. App. R. 49. Sean J. Brennan is an associate working in the firm’s Washington, D.C. office who currently is admitted to practice only in New York.
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
On May 3, 2023, the European Commission (the “Commission”) proposed a new directive[1] in the area of criminal law with the goal to harmonize corruption offenses, sanctions, related prevention and enforcement (the “Proposed Directive”). If adopted by the European Parliament and Council, the directive would significantly contribute to unifying and tightening rules across Europe. EU Member States would have to transpose that framework into national law within 18 months.[2] Since the Commission proposes “minimum rules concerning the definition of criminal offences and sanctions in the area of corruption, as well as measures to better prevent and fight corruption”[3], the Member States may go beyond the standards set out in the Proposed Directive and adopt even stricter rules in the area of corruption.
1. Key Takeaways
- The Commission suggests minimum standards to harmonize the definitions and sanctions for active and passive bribery both in the private and public sectors, as well as of related offenses such as “misappropriation”, “trading in influence”, “abuse of functions”, “obstruction of justice”, and “enrichment from corruption offences”.
- The Proposed Directive is based on a broad notion of public officials, not only covering EU officials, but also – across branches – functionaries of Member States, third country and international organizations and courts.
- The proposal reflects that certain elements of gifts and hospitality are socially more accepted in the area of private enterprise compared with interactions with state functionaries.
- If committed by a leading person, a legal person can be held liable for corruption offenses committed for its benefit.
- The Commission resorts to its usual terminology by requiring Member States to adopt “effective, proportionate and dissuasive” sanctions, both for natural persons and legal entities. Penalties for human beings may include imprisonment, the sanctions for legal entities may entail fines of no less than 5% of the total worldwide turnover. Further consequences include debarment or disqualification from commercial activities.
- Effective internal controls, ethics awareness, and compliance programs to prevent corruption are considered a mitigating factor, as well as the rapid and voluntary disclosure to the competent authorities.
- Jurisdiction attaches, (1) if the offense is committed in whole or in part in the territory of a Member State; (2) if the offender is a national of or has his or her habitual residence in a Member State; or (3) if the offense is committed for the benefit of a legal person established in the territory of a Member State.
2. Individual Criminal Liability
At its core, the Proposed Directive provides definitions of bribery in the public sector and the private sector; both in the active and passive alternative.
a) Bribery in the Public Sector
Section 7 of the Proposed Directive defines bribery in the public sector as such:
Member States shall take the necessary measures to ensure that the following conduct is punishable as a criminal offense, when committed intentionally:
(a) the promise, offer or giving, directly or through an intermediary, of an advantage of any kind to a public official for that official or for a third party in order for the public official to act or refrain from acting in accordance with his duty or in the exercise of that official’s functions (active bribery);
(b) the request or receipt by a public official, directly or through an intermediary, of an advantage of any kind or the promise of such an advantage for that official or for a third party, in order for the public official to act or to refrain from acting in accordance with his duty or in the exercise of that official’s functions (passive bribery).
The Proposed Directive is based on a broad notion of public officials, including not only (European) “Union officials,” but also national officials of Member States and of third countries, as well as any other person assigned and exercising a public service function in Member States or third countries, for an international organization or for an international court.[4] The definition of national officials is said to not only include persons holding executive, administrative or judicial offices, but also legislative office[5] (an area in which some countries such as Germany may have had some deficiencies in terms of combatting corruption[6]).
However, the Proposed Directive also contains elements that may, if interpreted broadly, limit the scope of the offense considerably. By way of example, the “advantage” to the public official or third party needs a connection with some performance of the public official in return, given that it must be “in order for the public to act or refrain from acting in accordance with his duty or in the exercise of that official’s functions”. This is arguably more restrictive than some current national laws that criminalize the granting or accepting of benefits without a specific compensation in return.[7]
b) Bribery in the Private Sector
The EU Commission also seeks to introduce an concept of bribery in the private sector
Member States shall take the necessary measures to ensure that the following conduct shall be punishable as a criminal offense, when committed intentionally and in the course of economic, financial, business or commercial activities:
(a) the promise, offer or giving, directly or through an intermediary, an undue advantage of any kind to a person who in any capacity directs or works for a private-sector entity, for that person or for a third party, in order for that person to act or to refrain from acting, in breach of that person’s duties (active bribery);
(b) the request or receipt by a person, directly or through an intermediary, of an undue advantage of any kind or the promise of such an advantage, for that person or for a third party, while in any capacity directing or working for a private-sector entity, to act or to refrain from acting, in breach of that person’s duties (passive bribery).[8]
In principle, this offense appears to be similarly conceptualized as bribery in the public sector. However, a remarkable feature is that this offense requires an “undue advantage” as opposed to a mere “advantage”. By suggesting this qualification, the Commission seems to reflect that certain elements of gifts and hospitality are socially more accepted in the area of private enterprise compared with interactions with state functionaries. Interestingly, the Proposed Directive does not contain a definition of “advantage”, let alone of an “undue advantage”, which may open the door for a broad interpretation of that element.
c) Further Offenses and Substantive Stipulations
The Proposed Directive would also impact national criminal laws, in that its Articles 9 to 13 require Members States to introduce or refine further offenses which form part of the fight against corruption, i.e. “misappropriation”, “trading in influence”, “abuse of functions”, “obstruction of justice”, and “enrichment from corruption offences”.
Member States are also requested to ensure that they can punish these offenses in cases of incitement, as well as aiding and abetting.[9] The Proposed Directive does not require Member States to criminalize “attempts” of bribery and passive bribery,[10] but this is an area where Member States may go beyond the Proposed Directive.[11]
3. Sanctions
With respect to punishment, the Commission resorts to its usual terminology by requiring Member States to adopt “effective, proportionate and dissuasive” criminal penalties, but also provides rather detailed specifications for the ranges of punishment.[12] Pursuant to the Proposed Directive, bribery in the public sector, as well as obstruction of justice, need to be punishable by a maximum term of at least six years. Bribery in the private sector is apparently deemed less grave, as the Commission foresees a maximum term of at least five years. Further legal consequences envisioned by the Proposed Directive entail, among others, fines, removal and disqualification from public office or the exercise of commercial activities in the context of which the offense was committed, and exclusions from access to public funding.[13]
4. Jurisdiction
In essence, the Proposed Directive foresees jurisdiction of the Member States over corruption offenses if one of three conditions apply:
- The offense is committed in whole or in part in the territory of a Member State;
- The offender is a national of or has his or her habitual residence in a Member State; or
- The offense is committed for the benefit of a legal person established in the territory of a Member State.[14]
This is arguably a similar framework to the version set out by the U.S. Foreign Corrupt Practices Act.[15] Practical enforcement would need to show whether extraterritorial enforcement of anti-corruption law by EU Member States or the European Public Prosecutor’s Office would gain a more significant role than in the past.
5. Corporate Liability / Relevancy of Compliance Programs and Internal Control Systems
The Proposed Directive prescribes that the Member States take necessary measures to ensure that legal entities can be “held liable” for any of such crimes.[16] This language is supposedly due to the fact that European legal orders vary significantly when it comes to “corporate crime”. Presumably against this background, the Proposed Directive takes a narrow approach, in that it requires that the offense be committed:
- for the benefit of a legal person;
- by a natural person within the legal person, acting either individually or as part of an organ of the legal person; and
- by having a leading position within the legal person, based on at least one of the following: A power of representation of the legal person; the authority to take decisions on behalf of the legal person; or the authority to exercise control within the legal person.
If a more subordinate employee committed a relevant offense, legal persons must be held liable if the lack of supervision or control by a leading person has made possible the commission of a crime by a person under his or her authority.[17]
In terms of sanctions for legal persons, the Proposed Directive stipulates that they need to include criminal or non-criminal fines of a maximum limit of no less than 5% of the total worldwide turnover of the legal person, including related entities, in the business year preceding the decision imposing the fine.[18] Further sanctions include the exclusion from entitlement to public benefits or aid; the temporary or permanent exclusion from public procurement procedures; temporary or permanent disqualification of that legal person from the exercise of commercial activities; the withdrawal of permits or authorizations to pursue activities in the context of which the offense was committed; the possibility for public authorities to annul or rescind a contract with the legal entity in the context of which the offense was committed; the placing of that legal person under judicial supervision; the judicial winding-up of that legal person; or the temporary or permanent closure of establishments which have been used for committing the offense.[19]
Article 18 of the Proposed Directive includes examples of aggravating and mitigating circumstances. A very relevant mitigating circumstance applies to a legal entity if it has implemented effective internal controls, ethics awareness, and compliance programs to prevent corruption prior to or after the commission of the offense.[20] The Proposed Directive is not more detailed on the specific requirement in this regard. A legal person can benefit from a further, arguably controversial, mitigating factor if it rapidly and voluntarily discloses the offense to the competent authorities and takes remedial measures.[21] This incentive forms part of a general international trend to encourage legal entities to inform prosecuting authorities of criminal offenses committed in its corporate environment.[22]
6. Prevention, Enforcement and Monitoring
The Commission goes considerably beyond merely harmonizing the substantive law, but aims through a variety of means to lay the ground for a comprehensive fight against corruption. For instance, the Proposed Directive sets out several Member State obligations to prevent corruption (such as raising public awareness).[23] It also introduces “specialized bodies”, both in the prevention and repression of corruption, to be established by the Member States,[24] and makes further provisions for resources, training, and investigative tools,[25] as well as cooperation between Member States and EU institutions[26].
_________________________
[1] Eur-Lex, Proposal for a Directive of the European Parliament and of the Council on combating corruption, replacing Council Framework Decision 2003/568/JHA and the Convention on the fight against corruption involving officials of the European Communities or officials of Member States of the European Union and amending Directive (EU) 2017/1371 of the European Parliament and of the Council, dated May 3, 2023, available under https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2023%3A234%3AFIN (last visited May 15, 2023).
[2] Article 29(1) of the Proposed Directive.
[3] Article 1 of the Proposed Directive (emphasis added).
[4] Article 2 no. 3 of the Proposed Directive.
[5] Article 2 no. 5 of the Proposed Directive.
[6] See 2022 Mid-Year FCPA Update / Covid-19 Mask Scandal.
[7] See, e.g. sections 331 and 333 of the German Criminal Code.
[8] Article 8 of the Proposed Directive.
[9] Articles 14(1) and (2) of the Proposed Directive.
[10] Article 14(3) of the Proposed Directive.
[11] By way of example, see sections 331(2), 332(1), and 334(2) of the German Criminal Code.
[12] Articles 15(1) and (2) of the Proposed Directive.
[13] Article 15(4) of the Proposed Directive.
[14] Article 20(1) of the Proposed Directive.
[15] 15 U.S. Code §§ 78dd-1 et seq.
[16] Article 16(1) of the Proposed Directive.
[17] Article 16(2) of the Proposed Directive.
[18] Article 17(2)(a) of the Proposed Directive.
[19] Article 17(2) of the Proposed Directive.
[20] Article 17(2)(b) of the Proposed Directive.
[21] Article 17(2)(c) of the Proposed Directive.
[22] See, e.g., Lisa Monaco, Memorandum of the U.S. Deputy Attorney General, September 15, 2022, p. 3.
[23] Article 3(1) of the Proposed Directive.
[24] Article 4 of the Proposed Directive.
[25] Articles 5, 6, and 23 of the Proposed Directive.
[26] Articles 20(2) and 24 of the Proposed Directive.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. If you wish to discuss any of the matters set out above, please contact the Gibson Dunn lawyer with whom you usually work, any member of Gibson Dunn’s White Collar Defense and Investigations or Anti-Corruption and FCPA practice groups in Germany, or the following authors in Munich:
Katharina Humphrey (+49 89 189 33 217, khumphrey@gibsondunn.com)
Andreas Dürr (+49 89 189 33-219, aduerr@gibsondunn.com)
Corporate Compliance / White Collar Matters
Ferdinand Fromholzer (+49 89 189 33 270, ffromholzer@gibsondunn.com)
Kai Gesing (+49 89 189 33 285, kgesing@gibsondunn.com)
Markus Nauheim (+49 89 189 33 222, mnauheim@gibsondunn.com)
Markus Rieder (+49 89 189 33 260, mrieder@gibsondunn.com)
Benno Schwarz (+49 89 189 33 210, bschwarz@gibsondunn.com)
Finn Zeidler (+49 69 247 411 530, fzeidler@gibsondunn.com)
Mark Zimmer (+49 89 189 33 230, mzimmer@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome
On April 28, 2023, the U.S. Department of Justice’s Consumer Protection Branch (CPB) released its second annual Recent Highlights Report.[1] The Report describes the Branch and its role at DOJ, and it reviews significant litigation and resolutions that occurred in 2022. Gibson Dunn will discuss the Report and other developments related to the Consumer Protection Branch in a complimentary webcast on Wednesday, May 31, from 12:00 p.m. to 1:00 p.m. EDT. You may register for the webcast here. The below alert also summarizes key aspects of the Report.
Consistent with last year’s publication,[2] the Report again highlights CPB’s growth, both in terms of its size and its widening scope of criminal and civil actions. With well more than 200 people (including more than 110 prosecutors), the Branch is the fastest-growing enforcement component at the Department of Justice. Indeed, the Branch currently is onboarding a large number of new prosecutors and would receive further enhancement through the President’s recent budget request.[3]
The Report also showcases CPB’s expanding relationships with agency partners. Those partners include various law enforcement agencies and consumer protection regulators, including the U.S. Food and Drug Administration (FDA), the Federal Trade Commission (FTC), the Drug Enforcement Administration (DEA), the Consumer Product Safety Commission (CPSC), and the National Highway Traffic Safety Administration (NHTSA), among others. Working with these partners, the Branch is expanding the use of criminal and civil penalty authorities to new enforcement spaces.
The Report flags several enforcement trends. With regard to consumer health and safety, it is clear that the Branch is dedicating particular attention to opioids, dietary supplements, tobacco products, and food safety.[4] The Branch also is notably focused on distributors of regulated products, with the Report describing actions and investigative efforts to hold distributors—defined in the Report as including e-commerce and social media companies—accountable for “allowing” products “to get into the [wrong] hands.”
As to consumer fraud and deceptive practices, the Report emphasizes the continuing expansion of the Branch’s partnership with the FTC, especially in civil penalty actions, which the Branch litigates on behalf of the FTC. With the FTC now referring to the Branch dozens of civil-penalty actions a year, the litigation of such actions has become a huge component of the Branch’s work and reflects a significant shift in how FTC-related actions are litigated. The Report also describes the Branch’s ongoing commitment to prosecuting schemes that target the elderly and other vulnerable populations.
* * *
The Report describes CPB’s work in three main areas: consumer health and safety enforcement, consumer fraud and deceptive practices enforcement, and the defense of federal consumer protection agencies. It also discusses new corporate compliance policies.
Consumer Health and Safety Enforcement
The Report highlights CPB’s work in enforcing the Federal Food, Drug, and Cosmetic Act (FDCA) and in combating the opioid epidemic. In the FDCA context, the Branch remains active in pursuing criminal and civil cases involving misbranded and adulterated drugs, medical devices, dietary supplements, biologics, food, and tobacco. The Report calls particular attention to the Branch’s resolution of a long-running civil action involving cigarette marketing that will result in corrective statements being published at approximately 200,000 retail locations throughout the country. The Report also reviews the Branch’s enforcement efforts involving unapproved nicotine products, unlawful dietary supplements, adulterated infant formula and other food, and misbranded drugs and medical devices. Food safety is an enforcement area emphasized in the Report, which states that the Branch is “[w]orking closely with the FDA and CDC” to pursue “civil and criminal actions against companies and individuals who fail to maintain sanitary facilities, distribute tainted food products, or make significant misrepresentations to customers or the public.” The Report also continues a trend of statements by the Branch highlighting its work to address clinical triad fraud,[5] with the Report describing multiple prosecutions advanced and convictions secured for alleged conduct related to the falsification of results, records, and other information.
The Report also notes the Branch’s strengthened efforts to combat the opioid epidemic. These efforts include nationwide civil actions under the Controlled Substances Act (CSA) related to the distribution and dispensing of prescription drugs. The efforts also include new actions to address the sharp rise in overdose deaths due to counterfeit pills laced with fentanyl. As noted in the Report:
“[T]he Branch has broadened its efforts to pursue corporate bad actors facilitating the manufacture, distribution, or sale of counterfeit pills. This includes investigating e-commerce sites and social media platforms that may be allowing traffickers to sell counterfeit pills to teens and young adults. Further, the Branch is investigating companies that may be allowing precursor chemicals and equipment to get into the hands of drug trafficking organizations.”
These “counterfeit pill initiatives” rely on the potential application of various provisions of the CSA and other laws that have not been frequently utilized. But that approach is consistent with the Branch’s work in prior years to use novel enforcement pathways in cases involving diverted prescription opioids, misbranded drugs, and hazardous products.
Further, the Report includes an interesting call out to the Branch’s work with CPSC and NHTSA. While no enforcement actions with those agencies are highlighted in the Report, their reference reflects increased collaboration with both agencies, especially with respect to criminal enforcement efforts.
Consumer Fraud and Deceptive Practice Enforcement
In the consumer fraud space, the Report notes a continued focus on transnational and complex consumer fraud schemes, as well as the enforcement of statutes administered by the FTC.
The Report makes clear that the Branch is continuing to collaborate more with the FTC, especially to advance actions seeking civil penalties for FTC rules or order violations. Under the FTC Act, the FTC must refer all actions seeking civil penalties to the Department of Justice for litigation. CPB receives and handles those referrals. In the wake of the Supreme Court’s decision in AMG Capital Management,[6] the FTC has increased dramatically the number of civil penalty referrals sent to the Branch. In fact, the Branch now receives dozens of referrals annually, requiring more than 40,000 hours of personnel time to litigate last year.
Highlighted in the Report are a $275 million judgment against a video game developer for allegedly collecting personal information in violation of Children’s Online Privacy Protection Act, and a $150 million civil penalty judgment against a social media company for allegedly failing to comply with data privacy provisions of a prior FTC order. The Report also notes that the Branch filed the first case under the FTC’s “Made in the USA” rule, multiple telemarketing cases, and other actions for unfair or deceptive practices. In addition, the Report highlights a case in which the Branch combined claims that a product violated both an FTC rule and the FDCA’s misbranding provisions—a combining of claims only possible because of the FTC’s referral of civil penalty actions to the Branch.
The Report also reviews CPB’s continued work fighting scams that target or disproportionately affect the elderly, immigrants, veterans, and vulnerable populations. That work includes the Branch’s coordination of global fraud-fighting efforts through the Department’s Transnational Elder Fraud Strike Force, which Attorney General Garland expanded to cover twenty federal districts.
Defensive Litigation
The Report includes a robust section discussing CPB’s enhanced efforts and capabilities to defend consumer protection agencies against challenges to their actions brought under the U.S. Constitution and the Administrative Procedure Act. Such challenges often involve the authorization or denial of medical device or drug approvals, the issuance of public health guidance, or product recalls. Some highlights noted in the Report include the Branch’s defense of the FDA’s orders denying marketing authorization for e-cigarette products, and its defense of the FDA in cases related to COVID-19 vaccines and treatments. Although not mentioned in the Report due to ongoing litigation, the Branch is also defending the FDA in challenges involving the drug mifepristone. The Branch’s defensive litigation work has expanded substantially over the past year and remains a space to watch.
Corporate Compliance
All of the Branch’s enforcement efforts will be informed by its new voluntary self-disclosure and monitor-selection policies, which we detailed in a previous alert in March of this year.[7] Those policies signal a desire to incentivize self-disclosure directly to CPB and to impose independent monitors more frequently. The Report explains that the policies are overseen by CPB’s Corporate Compliance and Policy Unit, which “helps to craft and enforce corporate resolutions,” including by working to “assess compliance programs, craft resolution terms, and ensure that defendants follow the compliance and reporting provisions of resolutions.”[8]
* * *
The Report makes clear that CPB remains one of the Department of Justice’s most active enforcers, using its unique ability to employ criminal and civil authorities to bring actions across a wide range of areas. Gibson Dunn has deep familiarity with CPB and experience in navigating actions involving it. We stand ready to assist clients engaging with the Branch.
___________________________
[1] United States Department of Justice Consumer Protection Branch, “Recent Highlights” (Apr. 2023), available at https://www.justice.gov/d9/2023-04/CPB%20Highlights.pdf.
[2] United States Department of Justice Consumer Protection Branch, “Recent Highlights” (Apr. 2022), available at https://www.justice.gov/file/1490441/download.
[3] See https://www.whitehouse.gov/wp-content/uploads/2023/03/jus_fy2024.pdf.
[4] See also United States Department of Justice, “Deputy Assistant Attorney General Arun G. Rao Delivers Remarks at the Food & Drug Law Institute’s (FDLI) 2021 Enforcement, Litigation and Compliance Conference” (Dec. 7, 2022), available at https://www.justice.gov/opa/speech/deputy-assistant-attorney-general-arun-g-rao-delivers-keynote-address-food-and-drug-law.
[5] See e.g. United States Department of Justice, “Deputy Assistant Attorney General Arun G. Rao Delivers Remarks at the Food & Drug Law Institute’s (FDLI) 2021 Enforcement, Litigation and Compliance Conference” (Dec 9, 2021), available at https://www.justice.gov/opa/speech/deputy-assistant-attorney-general-arun-g-rao-delivers-remarks-food-drug-law-institute-s.
[6] AMG Capital Management, LLC, et al. v. Federal Trade Commission, 593 US _ (2021).
[7] See Client Alert, Gibson Dunn, DOJ’s Consumer Protection Branch Announces New Corporate Enforcement Policies (March 28, 2023), https://www.gibsondunn.com/doj-consumer-protection-branch-announces-new-corporate-enforcement-policies/.
[8] United States Department of Justice Consumer Protection Branch, “Recent Highlights” (Apr. 2023), at 36, available at https://www.justice.gov/d9/2023-04/CPB%20Highlights.pdf.
The following Gibson Dunn lawyers prepared this client alert: Gus Eyler, Svetlana Gans, Nick Hanna, Ashley Rogers, Patrick Stokes, Sarah Hafeez, and Wynne Leahy.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding the issues discussed in this update. Please contact the Gibson Dunn lawyer with whom you usually work in the firm’s FDA and Health Care, Privacy, Cybersecurity and Data Innovation, or White Collar Defense and Investigations practice groups, or the authors:
FDA and Health Care Group:
Gustav W. Eyler – Washington, D.C. (+1 202-955-8610, geyler@gibsondunn.com)
Marian J. Lee – Washington, D.C. (+1 202-887-3732, mjlee@gibsondunn.com)
John D. W. Partridge – Denver (+1 303-298-5931, jpartridge@gibsondunn.com)
Jonathan M. Phillips – Washington, D.C. (+1 202-887-3546, jphillips@gibsondunn.com)
Privacy, Cybersecurity and Data Innovation:
S. Ashlie Beringer – Palo Alto (+1 650-849-5327, aberinger@gibsondunn.com)
Svetlana S. Gans – Washington, D.C. (+1 202-955-8657, sgans@gibsondunn.com)
Jane C. Horvath – Washington, D.C. (+1 202-955-8505, jhorvath@gibsondunn.com)
Ashley Rogers – Dallas (+1 214-698-3316, arogers@gibsondunn.com)
Alexander H. Southwell – New York (+1 212-351-3981, asouthwell@gibsondunn.com)
White Collar Defense and Investigations Group:
Stephanie Brooker – Washington, D.C. (+1 202-887-3502, sbrooker@gibsondunn.com)
Nicola T. Hanna – Los Angeles (+1 213-229-7269, nhanna@gibsondunn.com)
Charles J. Stevens – San Francisco (+1 415-393-8391, cstevens@gibsondunn.com)
Patrick F. Stokes – Washington, D.C. (+1 202-955-8504, pstokes@gibsondunn.com)
F. Joseph Warin – Washington, D.C. (+1 202-887-3609, fwarin@gibsondunn.com)
Debra Wong Yang – Los Angeles (+1 213-229-7472, dwongyang@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome
Gibson Dunn’s Public Policy Practice Group is closely monitoring developments regarding the infrastructure permitting debate in Congress. We offer this alert summarizing and analyzing the U.S. Senate Energy and Natural Resources Committee’s hearing on May 11, 2023, to help our clients prepare for potential changes in infrastructure permitting and environmental authorization laws. We are also available to help our clients arrange meetings on Capitol Hill to discuss permitting reform proposals or to share real-world examples of how the permitting process has affected them.
***
On May 11, 2023, the U.S. Senate Committee on Energy and Natural Resources (“ENR” or the “Committee”) held a hearing addressing the need to improve the federal infrastructure permitting process. Committee Chairman Joe Manchin (D-WV) repeatedly emphasized the importance of Congress passing a bipartisan permitting reform bill and promised, “We’re going to make something happen.” Other senators and witnesses alike concurred on the need for permitting reform to strengthen national security, address climate change, and support economic growth.
Witnesses included:
- Jason Grumet, Chief Executive Officer, American Clean Power Association;
- Rich Nolan, President and Chief Executive Officer, National Mining Association;
- Elizabeth Shuler, President, American Federation of Labor and Congress of Industrial Organizations (“AFL-CIO”); and
- Paul Ulrich, Vice President, Jonah Energy.
We provide a full hearing summary and analysis below. Of particular interest to clients, however:
- Chairman Manchin expressed his commitment to bipartisan permitting reform. He acknowledged the main proposals to date: his own bill, S. 1399, the Building American Energy Security Act of 2023; Committee Ranking Member John Barrasso’s S. 1456, the Spur Permitting of Under Developed Resources (“SPUR”) Act; Environment and Public Works Committee Ranking Member Shelley Moore Capito’s S. 1499, the Revitalizing the Economy by Simplifying Timelines and Assuring Regulatory Transparency (“RESTART”) Act; and H.R. 1, the Lower Energy Costs Act, which has passed the House. He also noted that Environment and Public Works (“EPW”) Committee Chairman Tom Carper will be offering his own proposal soon. Chairman Manchin argued that bipartisan reform will draw from all of these proposals.
- This hearing featured more comity than the EPW Committee’s hearing two weeks ago. Although the EPW members mostly agreed permitting reform is necessary, the Committee Democrats and several witnesses seemed concerned that permitting reforms may reduce community input. The ENR Committee members, however, all seemed to agree that permitting reform does not mean losing community participation or reducing environmental standards.
- Like the EPW Committee, ENR Chairman Manchin and Ranking Member Barrasso support developing permitting reform through regular order (meaning through the committee process, rather than a “gang”).
- Ranking Member Barrasso (R-WY) specified four requirements for any permitting bill that passes Congress. The bill: (1) must benefit the entire country, not a narrow range of special interests, limited projects, or specific technology. It must apply to all energy sources, including traditional and alternative energy; (2) include enforceable timelines for environmental authorizations; (3) limit legal challenges; and (4) stop the executive branch from “hijacking the permitting process to advance its own narrow and frequently extreme agenda.”
- Senator Angus King (I-ME) noted that when he was governor of Maine, he prioritized high environmental standards and a timely and predictable permitting process, arguing those two things are not inconsistent. As an Independent with practical experience from his time as governor, Senator King may well become a key player in the permitting reform effort.
Key substantive issues surrounding permitting reform raised in the hearing included: (1) the effectiveness of the FAST-41 permitting reforms; (2) the scope of permitting reform; (3) enforceable timelines and regulatory clarity; (4) litigation; (5) mining and critical minerals; (6) jobs and workforce; and (7) national security.
1. Effectiveness of FAST-41 Permitting Reforms
Several senators and witnesses commented on the effectiveness of the FAST-41 permitting reforms at reducing permitting timelines without harming environmental standards. Based on positive comments from both Republican and Democratic senators on the ENR and EPW committees, it seems likely that any bipartisan permitting reform proposal either will expand the FAST-41 program itself or apply FAST-41 principles, such as the designation of a lead agency for each project, deadline transparency and accountability, and expedited litigation timelines to more projects.
Chairman Manchin discussed the permitting reforms in his bill, which largely draw from FAST-41 principles, including limiting the length of environmental reviews; imposing enforceable timelines on agency reviews; requiring agencies to coordinate with one another and produce one coordinated review (a process known as “One Federal Decision”) rather than multiple, disparate reviews. He said he was “pleased” to see many of those ideas in Ranking Member Barrasso’s bill.
Senator Mark Kelly (D-AZ) commented that he supported efforts to permanent reauthorize FAST-41 last Congress, calling it a critical tool to help large projects navigate the permitting process. He acknowledged that the South32 Project became the first mining project to be covered by FAST-41 earlier this year.
Mr. Grumet also endorsed those reforms, calling FAST-41 and its Federal Permitting Improvement Steering Council “shockingly effective” at reducing permitting timelines.
2. Scope of Permitting Reform
One of the most significant areas of disagreement to date regarding permitting reform is which types of projects such reform should benefit. Many Democrats are focused on permitting reform for alternative energy projects and transmission lines and infrastructure. Republicans generally urge permitting reform for all infrastructure projects and support an all-of-the-above energy strategy, and they have concerns about taking away authority from state governments to permit transmission lines. Chairman Manchin, however, is trying to bridge that divide. At the hearing, he expressed concern about the substantial reduction in new natural gas infrastructure, as well as transmission infrastructure, as well as manufacturing and mining. He commented that “no matter what you want to build, it takes too long.”
Regarding transmission, Chairman Manchin explained that his bill recognizes that state governments have primary authority to site transmission lines, but offers reasonable improvements that would allow the Federal Energy Regulatory Commission (“FERC”) to step in when states cannot reach agreement after one year so long-distance interstate transmission projects can move forward. He suggested that his bill addresses Republican cost allocation concerns by ensuring that only those who receive electric benefits will pay for those benefits, and those payments will be proportionate. Senators Cindy Hyde-Smith (R-MS) and Josh Hawley (R-MO), though, expressed skepticism about proposals that yield more authority to the federal government over state approvals, and Senator John Hoeven (R-ND) raised opposition to policies that may distribute transmission costs unfairly.
Ranking Member Barrasso argued that Congress “cannot enact anything less than comprehensive reform,” pointing to his SPUR Act, which he said would revitalize the energy sector, hold the Secretary of Interior to her legal obligations regarding leasing on federal lands, and ensure access to minerals for renewable and battery technology. He asserted the bill would provide companies more predictable permitting processes for pipelines, liquid natural gas (“LNG”) facilities, and electric transmission lines. He invited Mr. Ulrich to testify regarding his company’s efforts to produce natural gas in an environmentally responsible manner.
Senators Maria Cantwell (D-WA) and Steve Daines (R-MT) both called for any bipartisan permitting reform proposal to incorporate their bill, S. 1521, to improve the licensing of non-federal hydropower projects. Senator Ron Wyden (D-OR) asked to be added as a co-sponsor during the hearing.
In his written testimony, Mr. Grumet supplied specific and detailed suggestions for Congress to enact transmission line permitting reform that would ensure regions can supply energy to each other across the country in an emergency. He argued that the current balkanized system leads to inefficient permitting and, ultimately, life-or-death emergencies when one region’s grid struggles. He proposed requiring states to conduct their own evaluations against FERC-supplied criteria. He also endorsed many of the FAST-41 principles and certain of the National Environmental Policy Act (“NEPA”) reforms contained in Chairman Manchin’s bill and Republican proposals, mainly focused on default timelines for steps in the environmental authorization process.
3. Enforceable Timelines and Regulatory Clarity
Ranking Member Barrasso made it clear that any bipartisan permitting bill must impose enforceable timelines on environmental authorizations and provide regulatory clarity regarding authorization requirements. Senator King also discussed the importance of firm deadlines.
Senator Daines contended that any permitting reform bill needs to address the Ninth Circuit’s decision in Cottonwood Environmental Law Center v. U.S. Forest Service regarding what actions trigger federal agencies’ obligation to reinitiate consultation with the U.S. Fish and Wildlife Service or the National Marine Fisheries Service. Senator Daines said that the Committee would be taking up his bipartisan legislation, S. 1540, addressing that issue next week.
Mr. Grumet testified that it seems like there is growing agreement that two years is an appropriate general timeline for permitting approval processes. Ms. Nolan argued that regulatory certainty is crucial for suppliers, contractors, and workers to make commitments to projects.
4. Litigation
Chairman Manchin expressed outrage over the eight years of litigation against the Mountain Valley Pipeline in West Virginia, which he noted has involved eight NEPA reviews and nine court cases in the U.S. Court of Appeals for the Fourth Circuit. Senator King said he supports limiting the length of time litigation takes. When he questioned Mr. Grumet about the appropriate length of time for a statute of limitations, however, Mr. Grumet demurred from committing to a specific number. He commented that the current six years is unnecessary; the FAST-41 change for certain projects to two years was “constructive”; and the Manchin legislation (at six months) is better.
Mr. Ulrich testified that the effects of litigation on oil and gas permitting are significant and argued any reform should expedite judicial review, limit venue shopping, and provide a reasonable statute of limitations for bringing an action against a project.
5. Mining and Critical Minerals
There was general consensus among senators and witnesses that the permitting process for mining needs reform. They recognized that critical mineral mining is crucial for U.S. national security and to combatting climate change.
Ranking Member Barrasso argued that it will be impossible to meet President Biden’s 2035 climate goals without a dramatic increase in our dependence on Russia and China for critical minerals unless Congress reforms the permitting process. He specifically focused on the need for miners to have access to federal lands to access critical minerals.
Senator Daines commented that permitting hard rock mining projects usually takes more than ten years, along with $10 billion in startup capital before producing any revenue—if they get through the permitting process. He commented that the Libby Exploration Project in Montana still has not finalized its permitting process because of litigation after 34 years.
Senator Catherine Cortez-Masto (D-NV) said that “everyone agrees” that we need to “get minerals and protect the environment,” citing the Biden administration’s focus on increasing domestic sources of critical minerals. She urged the mining industry to work harder on environmental issues and pressure bad actors, and also to collaborate with local stakeholders.
Senator Hawley observed that the U.S. labor protections are a “heck of a lot better” than those in China and the Democratic Republic of Congo, which currently provide much of the world’s mineral supply.
6. Jobs and Workforce
The hearing focused heavily on permitting reform’s impact on jobs and the economy. Both Republican and Democratic senators acknowledged that permitting reform is important for job creation.
In keeping with unions’ long support for permitting reform, Ms. Shuler testified that permitting reform is the AFL-CIO’s top priority, noting that every job in the energy and manufacturing sectors depend on permitting and siting. She claimed that permitting reform could “create more than a million good union jobs” while also bringing down emissions. She focused on the need to ramp up training to ensure workforce readiness when projects are approved.
Senator Kelly specifically focused on the importance of a faster permitting process to ensure that new jobs are available when older energy assets close.
Mr. Nolan offered that mining is a source of high-paying, stable, and often unionized jobs. He said mining directly employs 475,000 people in the United States with an average wage of $85,000.
7. National Security
Almost all of the senators and witnesses discussed the need to reduce reliance on China and Russia for critical minerals and manufacturing as a matter of national security. Chairman Manchin expressed frustration that the Biden administration does not focus on the national security purposes that motivated the Inflation Reduction Act, instead only discussing the climate change effects, and said he had been urging the administration to focus on its national security benefits. Senator King noted that 87 percent of lithium in electric vehicle batteries comes from China, which is a national security issue.
***
Senior members of Gibson Dunn’s Public Policy Practice Group have more than 40 years of combined experience on Capitol Hill. Our team includes former congressional staff and Administration officials who have significant experience tracking, developing, and implementing infrastructure permitting reform legislation and regulations. We also have strong working relationships with key members of Congress and Biden administration officials focused on federal permitting reform.
Our team is available to assist clients through strategic counseling; real-time intelligence gathering on federal permitting reform legislation; developing and advancing policy positions; drafting legislative text; shaping messaging; and lobbying Congress. We also work with clients to craft regulatory comment letters; advocate before executive branch agencies; and navigate legislative and regulatory changes to federal infrastructure permitting laws.
The following Gibson Dunn lawyers assisted in preparing this alert: Michael D. Bopp, Roscoe Jones Jr., David Fotouhi, Amanda Neely, and Daniel P. Smith.*
Gibson, Dunn & Crutcher’s lawyers are available to assist in addressing any questions you may have regarding these issues. Please contact the Gibson Dunn lawyer with whom you usually work in the firm’s Public Policy or Environmental Litigation and Mass Tort practice groups, or the following authors:
Michael D. Bopp – Co-Chair, Public Policy Group, Washington, D.C. (+1 202-955-8256, mbopp@gibsondunn.com)
Roscoe Jones, Jr. – Co-Chair, Public Policy Group, Washington, D.C. (+1 202-887-3530, rjones@gibsondunn.com)
David Fotouhi – Washington, D.C. (+1 202-955-8502, dfotouhi@gibsondunn.com)
Amanda H. Neely – Washington, D.C. (+1 202-777-9566, aneely@gibsondunn.com)
Daniel P. Smith* – Washington, D.C. (+1 202-777-9549, dpsmith@gibsondunn.com)
*Daniel P. Smith is of counsel working in the firm’s Washington, D.C. office who is admitted only in Illinois and practicing under supervision of members of the District of Columbia Bar under D.C. App. R. 49.
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome
Decided May 11, 2023
Percoco v. United States, No. 21-1158
Today, the Supreme Court overturned a wire fraud conviction based on an honest-services theory. The Court reasoned that while such a theory can potentially cover private persons, it does not extend to all private persons. The jury instructions given in this case were too vague because they failed to define the intangible right to honest services with sufficient definiteness.
Background: Joseph Percoco resigned from his position in New York state government to serve in a private capacity as then-Governor Andrew Cuomo’s campaign manager. Despite lacking an official government position, he continued to wield significant influence in the Cuomo administration. Recognizing that, an acquaintance paid Percoco $35,000 to have him pressure a state agency to drop a requirement that would have forced the acquaintance’s company to enter a costly agreement with a local union.
Federal prosecutors indicted Percoco under the honest-services component of the federal wire-fraud statute, which proscribes “depriv[ing] another of the intangible right of honest services.” 18 U.S.C. § 1346. The statute is typically invoked in public-corruption cases involving public officials who take money in exchange for exercising their official power. But the Second Circuit concluded that the statute is broad enough to encompass Percoco’s situation: a private citizen who takes money in exchange for wielding his substantial influence over government officials to persuade those officials to exercise their official powers in a certain way.
Issue: Whether private citizens can be convicted of depriving the public of honest services.
Court’s Holding:
Potentially yes, because there is no absolute rule that would preclude convicting a private citizen under an honest-services theory (such as where a private citizen has become an actual agent of the government). But the conviction at issue could not be upheld because the jury instructions were too vague.
“‘[T]he intangible right of honest services’ codified in § 1346 plainly does not extend a duty to the public to all private persons.”
Justice Alito, writing for the Court
What It Means:
- Today’s decision is one in a line of cases, including McDonnell v. United States, 579 U.S. 550 (2016),and Skilling v. United States, 561 U.S. 358 (2010), addressing expansive uses of the honest-services theory of wire fraud, and the Court’s holding will make it more difficult for prosecutors to assert such theories as to private citizens such as lobbyists.
- Today’s opinion suggests the government should focus on “heartland” cases of honest-services fraud, including when a private individual acts as the government’s “actual agent” and therefore owes a fiduciary duty to both the government and the public.
- Justice Gorsuch, joined by Justice Thomas, concurred in the judgment only. Justice Gorsuch would have held that the honest-services fraud statute is void for vagueness because it fails to provide the fair notice that due process requires. After today’s opinion, Justice Gorsuch wrote, “we now know a little bit more about when a duty of honest services does not arise, but we still have no idea when it does.”
The Court’s opinion is available here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
Related Practice: White Collar Defense and Investigations
| Stephanie Brooker +1 201.887.3502 sbrooker@gibsondunn.com |
F. Joseph Warin +1 202.887.3609 fwarin@gibsondunn.com |
|
| Chuck Stevens +1 415.393.8391 cstevens@gibsondunn.com |
Nicola T. Hanna +1 213.229.7269 nhanna@gibsondunn.com |
Decided May 11, 2023
Ciminelli v. United States, No. 21-1170
Today, the Supreme Court unanimously rejected the “right-to-control” theory of wire fraud, holding that potentially valuable economic information necessary to make discretionary economic decisions does not constitute “property” for purposes of the federal wire-fraud statute.
Background: In 2012, New York Governor Andrew Cuomo kicked off “Buffalo Billion,” a billion-dollar economic-development program. The state selected developers for the project through an Alain Kaloyeros-run nonprofit entity that solicited bids from contractors. Louis Ciminelli’s construction company submitted a bid and won a $750 million development contract. It was later revealed that Kaloyeros and Ciminelli had worked together to rig the bidding process in favor of Ciminelli’s bid. In 2018, Ciminelli and Kaloyeros were indicted for wire fraud.
The federal wire-fraud statute proscribes making false statements to obtain money or property. The trial court instructed the jury that “property” includes intangible interests, including the right to control the use of one’s assets. It further instructed the jury that depriving another of potentially valuable economic information violates the wire-fraud statute. The jury convicted Ciminelli and Kaloyeros. On appeal, the Second Circuit affirmed, upholding what it referred to as the “right-to-control” theory of wire fraud.
Issue: Whether depriving someone of potentially valuable economic information is a deprivation of “money or property” for purposes of the federal wire-fraud statute.
Court’s Holding:
No. The Court concluded that valuable economic information needed to make discretionary economic decisions is not a traditional property interest and does not constitute “property” for purposes of the federal wire-fraud statute, and therefore the “right-to-control” theory cannot form the basis for a conviction under the federal fraud statutes.
“Because ‘potentially valuable economic information’ ‘necessary to make discretionary economic decisions’ is not a traditional property interest, we now hold that the right-to-control theory is not a valid basis for liability under §1343.”
Justice Thomas, writing for the Court
What It Means:
- The Court rejected the notion that allegedly false statements made during contract negotiations that lead to no harm to a traditional property interest can form the basis for criminal liability under the federal fraud statutes. This holding should assuage the fears of contracting parties who, under the Second Circuit’s “right-to-control” theory, would risk criminal liability for alleged misstatements made during contract negotiations even where those misstatements lead to no harm to a traditional property interest.
- This decision is the latest in a series of cases in which the Court has rejected novel and expansive readings of federal fraud statutes in state and local public corruption cases. E.g., Kelly v. United States, 140 S. Ct. 1565 (2020); McDonnell v. United States, 579 U.S. 550 (2016). These decisions underscore the Court’s reluctance to over-criminalize common behavior and over-federalize traditionally state matters—particularly in cases touching on state and local politics.
- The Court also rejected the government’s request to uphold Ciminelli’s conviction on the alternative ground that the evidence was sufficient to establish wire fraud under a traditional property fraud theory, because the government relied exclusively on the right-to-control theory in indicting the defendants, obtaining their convictions, and prevailing in the Second Circuit.
- The decision will likely discourage further efforts on the part of prosecutors to base federal fraud cases on abstract injuries and instead will encourage them to focus on proving that alleged victims of fraud lost money or property. The government itself conceded before the Supreme Court that the “right-to-control theory” was erroneous, signaling a preemptive retreat from these types of prosecutions before the Court’s decision was even announced.
The Court’s opinion is available here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
Related Practice: White Collar Defense and Investigations
| Stephanie Brooker +1 201.887.3502 sbrooker@gibsondunn.com |
F. Joseph Warin +1 202.887.3609 fwarin@gibsondunn.com |
|
| Chuck Stevens +1 415.393.8391 cstevens@gibsondunn.com |
Nicola T. Hanna +1 213.229.7269 nhanna@gibsondunn.com |
Decided May 11, 2023
National Pork Producers Council v. Ross, No. 21-468
Today, the Supreme Court held in a fractured decision that California’s ban on the sale of pork that comes from pigs that were raised in a “cruel manner,” regardless of where the pigs are raised, does not violate the dormant Commerce Clause.
Background: In 2018, California voters approved Proposition 12, which prohibits selling pork in California if the pigs were housed in a “manner that prevents the animal from lying down, standing up, fully extending [its] limbs, or turning around freely.”
The National Pork Producers Council and the American Farm Bureau Federation sued, alleging that Proposition 12 violates the dormant Commerce Clause, which they argued bars state legislation that (i) discriminates against out-of-state interests, (ii) has impermissible extraterritorial effects, or (iii) imposes a clearly excessive burden on interstate commerce when compared to the putative local benefits. The plaintiffs did not argue that Proposition 12 discriminated against out-of-state interests. Instead, they relied exclusively on its extraterritorial effects and its burden on interstate commerce.
The district court dismissed the complaint and the Ninth Circuit affirmed. The Ninth Circuit held a state law has impermissible extraterritorial effects only if it “dictate[s] the price of a product” or “tie[s] the price of in-state products to out-of-state prices”—which Proposition 12 did not do. Acknowledging that the Supreme Court has “not provided a clear methodology for comparing in-state benefits and out-of-state burdens” to assess a law’s burden on interstate commerce, the Ninth Circuit nevertheless held that Proposition 12’s alleged increase in costs to businesses and consumers was not a constitutionally significant burden on interstate commerce.
Issue: Whether plaintiffs stated a plausible claim that Proposition 12 violates the dormant Commerce Clause because it has impermissible extraterritorial effects or places an undue burden on interstate commerce.
Court’s Holding:
No. The Court’s core dormant Commerce Clause precedents focus on state laws that discriminate against out-of-state commerce, which a law banning the sale in California of pork that was raised in a “cruel manner” does not do, and the plaintiffs failed to allege a plausible claim under Pike v. Bruce Church, Inc., 397 U.S. 137 (1970).
“While the Constitution addresses many weighty issues, the type of pork chops California merchants may sell is not on that list.”
Justice Gorsuch, writing for the Court
What It Means:
- The Court’s majority opinion underscores that the focus of the dormant Commerce Clause is on those state laws that discriminate against out-of-state commerce.
- The Court’s decision was highly fractured, as parts of Justice Gorsuch’s opinion discussing Pike were joined only by a plurality of the Court, and multiple justices wrote separate opinions. Justice Kavanaugh’s opinion attempts to outline the controlling rule, and suggests “that properly pled dormant Commerce Clause challenges under Pike to laws like California’s Proposition 12 (or even to Proposition 12 itself) could succeed in the future—or at least survive past the motion-to-dismiss stage.”
- The Court intimated that several other constitutional provisions may provide a stronger basis for challenging state laws that affect out-of-state commerce, including the Full Faith and Credit Clause, the Import-Export Clause, the Privileges and Immunities Clause, and the “principle inher[ing] in the very structure of the Constitution, which ‘was framed upon the theory that the peoples of the several states must sink or swim together.’”
The Court’s opinion is available here.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding developments at the Supreme Court. Please feel free to contact the following practice leaders:
Appellate and Constitutional Law Practice
| Allyson N. Ho +1 214.698.3233 aho@gibsondunn.com |
Thomas H. Dupree Jr. +1 202.955.8547 tdupree@gibsondunn.com |
Julian W. Poon +1 213.229.7758 jpoon@gibsondunn.com |
| Lucas C. Townsend +1 202.887.3731 ltownsend@gibsondunn.com |
Bradley J. Hamburger +1 213.229.7658 bhamburger@gibsondunn.com |
Brad G. Hubbard +1 214.698.3326 bhubbard@gibsondunn.com |
We are pleased to provide you with Gibson Dunn’s ESG monthly updates for April 2023. This month our update covers the following key developments. Please click on the blue links below for further details.
1. ISSB proposing to give relief on Scope 3 emissions and certain other data points in first year of reporting against S1 and S2
The International Sustainability Standards Board (ISSB) is proposing to extend reliefs available to support companies applying the ISSB’s first two Standards—S1 (general requirements) and S2 (climate).
The relief will enable companies to focus initial efforts on ensuring they meet investor information needs around climate change and means companies can prioritise putting in place reporting practices and structures to provide high-quality information about climate-related risks and opportunities in the first year of reporting using the ISSB Standards.
Companies will then need to provide full reporting on sustainability-related risks and opportunities, beyond climate, from the second year. This follows the view from investors that disclosures around climate-related risks and opportunities are the most urgent.
2. Launch of Venture Climate Alliance
The Venture Climate Alliance (VCA), an organization created by leading global venture capital firms to define, facilitate, and realize net zero-aligned pathways for early-stage investments, launched on April 25, 2023 with a goal to build a robust movement within the venture industry to combat climate change. CA members have committed to supporting a rapid, global transition to net zero or negative carbon emissions by 2050 or sooner, and will take specific, near-term steps to achieve this goal, both within their respective firms and in their roles as investors and advisors to their portfolio companies.
As one of the first institutional touchpoints between capital markets and early-stage innovation, venture capital investors have helped thousands of new companies from initial development to commercialization and scale. The VCA provides a forum through which member firms will develop best practices for collecting, interpreting and reporting carbon emissions, and climate impact data, as well as tools and guidance to help to overcome barriers associated with aligning early-stage investments with net zero goals.
3. G7 sets collective targets on renewable energy
The Group of Seven wealthy nations (G7) recently issued a Communique setting out ambitious collective goals to increase their solar power and offshore wind capacity. Their aim is to expedite the development of renewable energy and hasten the phase-out of fossil fuels. However, they did not endorse a 2030 deadline for ending the use of coal, instead leaving room for continued investment in gas. The members committed to boosting offshore wind capacity by 150 gigawatts and solar capacity by more than 1 terawatt by 2030. They also agreed to speed up the phase-out of fossil fuels without capturing CO2 emissions, with a target of achieving net-zero energy systems by 2050 at the latest. The G7 nations also pledged to take practical and timely measures to accelerate the phase-out of unabated coal power generation.
While Canada and some other G7 members support ending the use of unabated coal-fired power by 2030, others are still exploring ways to achieve that goal. The G7 countries acknowledged that renewable energy and energy security are compatible goals and committed to reaching a shared target for 2050. They also pledged to reduce additional plastic pollution to zero by 2040, ten years earlier than previously planned.
1. Northern Ireland faces legal action over new gas storage project
Northern Ireland’s decision to sign off an undersea project that could provide a quarter of the UK’s gas storage capacity will be challenged in the High Court in Belfast over environmental concerns in an action brought by Friends of the Earth Northern Ireland and local campaign group No Gas Caverns. It is believed to be the first case of its kind where the courts have had to grapple with the implications of climate change in the context of government decisions.
A judicial review hearing will start in the week commencing 8 May 2023, where lawyers will argue that the Northern Ireland Department of Agriculture, Environment and Rural Affairs (DAERA) failed to properly consider the environmental implications of the project when granting permission for the Islandmagee Gas Storage development.
2. Financial Conduct Authority to lead first GFIN Greenwashing TechSprint
On 11 April 2023, the UK Financial Conduct Authority (FCA) announced that it would be leading the Global Financial Innovation Network (GFIN)’s first ever virtual Greenwashing TechSprint. The TechSprint will be hosted on the FCA’s Digital Sandbox and aims to bring together international regulators, firms and innovators to address sustainable finance as a collective priority. UK-based firms are invited to apply from 17 April 2023.
The GFIN is a group of over 80 international organisations committed to supporting financial innovation in the interest of consumers. The GFIN’s Co-ordination Group, which sets the overall direction, strategy and annual work programme, is currently chaired by the FCA.
In light of the growing number of investment products marketed as ‘green’ or making wider sustainability claims, the FCA wishes to ensure consumers and firms can trust that products have the sustainability characteristics they claim to have. The objective of the TechSprint is to develop a tool or solution that can help regulators and the market effectively tackle the risks of greenwashing in financial services.
There are currently 13 international regulators that have signed up to participate in the TechSprint. The TechSprint will launch on 5 June 2023 and will run for 3 months, ending with a showcase day in September 2023.
3. The FCA’s view of Green Mortgages
Green mortgages – a mortgage which includes an incentive for people to either purchase an energy efficient property or improve the energy efficiency of an existing property – have a growing role to play in decarbonising the UK’s housing stock by helping borrowers to improve the energy efficiency of their homes. The incentives could be a discount to the fixed rate or a cash rebate after completion of agreed improvements.
In a recent speech by the FCA’s Director of Retail Banking emphasised the role that lenders have in this endeavour and flagged the risk of them missing their decarbonisation targets if they don’t evolve their support for homeowners to enhance energy efficiency. Brokers should see an increase in the availability of green mortgage products and innovation and they have a key role to play in helping borrowers navigate a complex and nuanced landscape in terms of green home finance.
Equally, there are certain risks in requiring lenders to make more green mortgages that could lead to greenwashing if the majority of loans are made to newer, and already efficient, homes or too much innovation such that the number of products available outstrips demand from consumers.
The FCA has also made it clear that lenders need to deliver products that are ethical, socially responsible and green and meet expectations of the consumers based on the advertising or marketing of the products.
1. EBA consultation on benchmarking of diversity practices and policies
On 24 April 2023, the European Banking Authority (EBA) published a consultation paper on draft guidelines on the benchmarking of diversity practices including diversity policies and gender pay gap under the Capital Requirements Directive IV (CRD IV) and the Investment Firms Directive (IFD), addressed to Member State competent authorities (NCAs).
The benchmarking of diversity practices will allow NCAs to monitor diversity trends over time, including the identification of common practices for diversity policies and information on the gender pay gap at the level of the management body. The aspects of diversity that will be analysed concern the gender, age, educational and professional background as well as the geographical provenance of members of the management bodies. The benchmarking of diversity practices should be based on a representative sample of institutions and investment firms.
The EBA will analyse the diversity practices, including diversity policies and the gender pay gap and publish a benchmarking report at the EU level, including a country-by-country analysis every three years. The data is not collected annually as the composition of the management bodies is not expected to change significantly in the short term, but should change in the longer term through taking appropriate measures within institutions and investment firms.
The EBA plans that the first data on the diversity practices under the guidelines should be reported in 2025 with a reference date of 31 December 2024, including in situations where the financial year differs from the calendar year.
2. European Council adopts new rules on pay transparency
The Council has adopted new rules to combat pay discrimination and help close the gender pay gap in the EU. Under the pay transparency directive, EU companies will be required to share information about how much they pay women and men for work of equal value, and take action if their gender pay gap exceeds 5%. Once in the role, workers will be entitled to ask their employers for information about average pay levels, broken down by sex, for categories of employees doing the same work or work of equal value. They will also have access to the criteria used to determine pay and career progression, which must be objective and gender neutral. The new directive also includes provisions on compensation for victims of pay discrimination and penalties, including fines, for employers who break the rules.
3. Parliament adopts new law to fight global deforestation
The European Parliament adopted a new EU law on 19 April 2023 that will ban imports deemed to be driving deforestation. The legislation, which has to get final approval from the European Union’s member countries, would apply to coffee, cocoa, soy, timber, palm oil, cattle, printing paper and rubber, and derived products, coming from countries around the world.
Imports that come from land that was deforested after 31 December 2020 will be prohibited in the EU market. Companies sending such merchandise to Europe will have to show a certificate guaranteeing they do not come from such zones, with checks conducted on a sliding scale according to how high risk the exporting country is ranked. Companies will also have to verify that these products comply with relevant legislation of the country of production, including on human rights, and that the rights of affected indigenous people have been respected.
1. Biden Executive Order – environmental justice
On 21 April 2023, President Biden signed the executive order “Revitalising Our Nation’s Commitment to Environmental Justice for All“, charging all federal agencies with a duty to pursue environmental justice. The executive order directs federal agencies to thoroughly review environmental and health impacts on communities. Under the new requirements, federal agencies must notify communities if toxic substances are released and host public meetings to inform local residents of any resulting health risks.
Increasing concerns about environmental justice come in the wake of the East Palestine train derailment on 4 February 2023, which resulted in the uncontrolled release of hazardous materials into the surrounding area. Following this incident, the Biden-Harris administration has growingly focused on the disproportionate impact of climate change and pollution on lower-income and otherwise disadvantaged communities. This executive order seeks to reduce the burden on such communities by requiring federal agencies to assess the “cumulative impacts” of an area’s environmental and health problems when making decisions on new facilities or projects.
Under the executive order, President Biden has established a new Office of Environmental Justice within the White House Council on Environmental Quality, which will oversee federal agencies’ efforts on environmental justice. In conjunction with this, the White House Council on Environmental Quality will publish an Environmental Justice Scorecard monitoring federal agencies’ progress.
2. Environmental Protection Agency announces proposal on new auto pollution limits
The Environmental Protection Agency (EPA) plans to release its most stringent new emission standards for cars and light trucks. Sources familiar with the matter indicate that the rules are intended to ensure that electric vehicles (EVs) make up 54% to 60% of all new cars sold in the US by 2030, and 64% to 67% by 2032. This aligns with the U.S. President Joe Biden’s goal of EVs accounting for at least 50% of new vehicle sales by 2030.
The EPA’s proposal follows California air regulators’ vote last year to ban the sale of new gasoline-powered cars by 2035 and set interim targets for phasing out these cars. California aims to have 70% of zero-emissions vehicles in all new car sales by 2030, and other states are also planning to adopt California’s rules, driving a shift to EVs. Unlike California’s policy, which directly targets vehicle sales, the EPA intends to implement increasingly stringent greenhouse gas emissions rules for automakers to follow from 2027 to 2032, thus promoting the electrification of the industry. In addition, the US Treasury Department released new rules on April 18 that will provide federal tax credits of up to USD 7,500 to buyers of certain EVs.
3. DOE report on CO2 management in US and steps needed to achieve net zero commitments by 2050
On April 24, the Department of Energy published a new report on carbon management, as part of its ongoing series of Pathways to Commercial Liftoff Reports. In particular, the report indicates the United States will need to rapidly accelerate investment in hydrogen, nuclear and long-duration energy storage in order to meet its commitment to obtaining net-zero emission by 2050, from an approximate USD 40 billion to USD 300 billion by 2030. In addition, the report states that the United States will need to store c.400–1,800 million tonnes of CO2 annually.
1. HKSE consultation paper on enhancements to climate-related disclosures
On 14 April 2023, the Hong Kong Stock Exchange published a consultation paper on enhancements to its climate-related disclosures regime, aiming to achieve closer alignment with the International Sustainability Standards Board Climate Standard, expected to be published before the end of June 2023.
At present, Appendix 27 to the Hong Kong Stock Exchange Listing Rules requires companies to disclose climate-related information on a “comply or explain” basis; the proposed changes would mandate companies to make those disclosures. Listed companies would also be subject to additional climate-related disclosure obligations under the revised Appendix 27. These relate to governance, strategy, risk management, and metrics and targets. Of particular note, an issuer would be required to disclose any climate-related risks and opportunities identified; any strategies prepared in response to those climate-related risks and opportunities; how the business is placed to prepare for and respond to climate-related changes; and any current and anticipated financial effects of climate-related risks.
It is anticipated that the revised HKSE Listing Rules and Appendix 27 will come into effect on 1 January 2024, subject to the responses to the consultation, due by 14 July 2023.
2. New Australian vehicle fuel efficiency standard
Australia is set to introduce fuel efficiency standards as part of its new national electric vehicle (EV) strategy, in a bid to catch up with other developed economies in promoting EV adoption. According to Energy Minister Chris Bowen, the new standards will stipulate the amount of carbon dioxide a car could produce while running. Details of the new EV strategy will be finalized in the coming months The Department of Infrastructure, Transport, Regional Development and Communications published a consultation paper on 19 April 2023 . Australia’s Electric Vehicle Council (EVC) has welcomed the move and stressed the need for strict standards, warning that Australia will “remain the world’s dumping ground for dated high-emission vehicles.”
Australia was the only developed country, aside from Russia, that did not have or was not setting fuel efficiency standards. The lag in environmental rules has made it difficult for EVs and low-emission cars to compete with dirtier and less efficient vehicles in Australia. In 2022, only 3.8% of cars sold in Australia were electric, while the proportion stood at 15% in the UK and 17% in Europe. Bowen acknowledged the need to boost the construction of EV charging facilities. As of December 2022, the country had only 4,900 public chargers at fewer than 2,400 sites. To address this, a policy requiring the installation of one fast charger every 150 kilometers on the highway will soon be implemented, according to Bowen, for the convenience of 83,000 EV drivers on Australian roads.
3. India extends waiver of transmission fees for green hydrogen plants
India will exempt transmission fees for renewable power used in hydrogen manufacturing plants built up before January 2031, extending a waiver previously available for projects set up before July 2025. According to sources familiar with the matter, the move will enable more green hydrogen projects to qualify for the 25-year waiver of transmission charges, thus reducing the manufacturing costs. A government official explained that large-scale hydrogen and ammonia projects will require three to four years to construct, which means that many of them may not come into operation by June 2025.
India aims to become the world’s cheapest producer of green hydrogen – hydrogen produced by splitting water using electricity from renewables. It plans to bring down the manufacturing cost from current levels of USD 4-5 per kg to between USD 1-1.5 per kg. According to industry estimates, renewable energy, including transmission, accounts for 65%-70% of the cost of producing green hydrogen. Therefore, the government official stated that every one rupee decrease in renewable energy costs would result in a 60 Indian rupee (USD 0.73) reduction in the cost of green hydrogen. The inter-state transmission charges range from 1-2 rupees per unit of power transmitted. Besides waiving transmission fees, the Indian government will provide green hydrogen fuel producers with incentives worth at least 10% of their costs, under a USD 2.11bn program called “Strategic Interventions for Green Hydrogen Transition (SIGHT) Program”. The scheme, effective June 2023, is intended to produce affordable green hydrogen and reduce greenhouse gas emissions.
Please let us know if there are topics that you would be interested in seeing covered in future editions of the monthly update.
Warmest regards,
Susy Bullock
Elizabeth Ising
Perlette M. Jura
Ronald Kirk
Michael K. Murphy
Selina S. Sagayam
Environmental, Social and Governance Practice Group Leaders, Gibson Dunn & Crutcher LLP
The following Gibson Dunn lawyers prepared this client update: Selina Sagayam, Charlie Osborne, Patricia Tan Openshaw, Grace Chong, and Elizabeth Ising.
Gibson Dunn lawyers are available to assist in addressing any questions you may have regarding these developments. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any leader or member of the firm’s Environmental, Social and Governance practice group:
Environmental, Social and Governance (ESG) Group Leaders and Members:
Susy Bullock – London (+44 (0) 20 7071 4283, sbullock@gibsondunn.com)
Elizabeth Ising – Washington, D.C. (+1 202-955-8287, eising@gibsondunn.com)
Perlette M. Jura – Los Angeles (+1 213-229-7121, pjura@gibsondunn.com)
Ronald Kirk – Dallas (+1 214-698-3295, rkirk@gibsondunn.com)
Michael K. Murphy – Washington, D.C. (+1 202-955-8238, mmurphy@gibsondunn.com)
Patricia Tan Openshaw – Hong Kong (+852 2214-3868, popenshaw@gibsondunn.com)
Selina S. Sagayam – London (+44 (0) 20 7071 4263, ssagayam@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome.
On 4 May 2023, the Hong Kong Court of Final Appeal (the “CFA”) handed down its judgment in Guy Kwok-Hung Lam v Tor Asia Credit Master Fund LP [2023] HKCFA 9[1], putting an end to the age-old debate on the effect of an exclusive jurisdiction clause (“EJC”) in the insolvency context.
The CFA upheld the Court of Appeal’s (the “CA”) decision (by majority) to dismiss the bankruptcy petition. The CFA endorsed the approach that in an ordinary case where the underlying dispute of the petition debt was subject to an EJC, the court should dismiss the petition unless there are strong reasons for the court to decide otherwise.
1. Background
The dispute concerned a loan advanced by Tor Asia Credit Master Fund LP (the “Petitioner”), pursuant to a Credit and Guaranty Agreement (the “Agreement”), to a company (the “Borrower”) controlled by Mr. Guy Kwok-Hung Lam (the “Debtor”), whereby the Debtor agreed to provide a guarantee, as primary obligor, to pay in full of all amounts due and owed without any demand or notice. The Agreement contained an EJC in favour of the New York courts in relation to “all proceedings arising out of or in relation to” the Agreement.
The Agreement was subsequently amended and the maturity of the loan was extended. Notwithstanding that, the Borrower was still unable to make repayment. The Petitioner then presented a bankruptcy petition in Hong Kong against the Debtor. The Debtor resisted the petition and argued that there was no event of default and that, pursuant to the EJC, the Petitioner was required to bring proceedings in the New York courts first to establish the Debtor’s liability.
The Court of First Instance (the “CFI”) granted the bankruptcy order, on the basis that the Debtor was unable to demonstrate a bona fide dispute on substantial grounds in relation to the petition debt[2]. The CA allowed the Debtor’s appeal and dismissed the bankruptcy petition[3]. The CA held that if the dispute concerning the underlying debt fell within the scope of an EJC, the bankruptcy petition should not be allowed to proceed without strong reasons.
The Petitioner appealed to the CFA on the proper approach that Hong Kong courts should adopt in a bankruptcy petition where the dispute concerning a debt is subject to an EJC.
2. The CFA’s decision
The CFA unanimously dismissed the appeal and affirmed the decision of the CA.
(i) Jurisdiction and powers of the CFI
The Petitioner contended that parties could not contract out of the insolvency legislation and in these proceedings different considerations were to be taken into account from those involving the upholding of EJCs in private actions. It was argued that to give presumptive weight to EJCs was to erode and undermine the domestic insolvency regime.
Whilst the CFA confirmed that the CFI’s jurisdiction in a bankruptcy matter was conferred by the Bankruptcy Ordinance (Cap. 6), and was not amenable to exclusion by contract, i.e. the parties’ agreement not to invoke the jurisdiction of the CFI had no effect on its jurisdiction, it held that the parties’ agreement to refer their disputes to a foreign court informed the CFI’s discretion as to whether to exercise its jurisdiction.
The CFA observed that the CFI might exercise its discretion to decline jurisdiction in certain classes of cases, such as where the issue of forum non conveniens was raised or where the dispute in a particular action was covered by an arbitration agreement or an EJC.
(ii) The discretion to decline jurisdiction in bankruptcy
Having found that the CFI had the power to decide whether to exercise its jurisdiction, the CFA further held that the determination of whether the debt was bona fide disputed on substantial grounds was a threshold question which might or might not be engaged when the court decided whether to exercise its bankruptcy jurisdiction.
The CFA noted that in the event that the parties had agreed to have all their disputes under an agreement giving rise to the debt determined exclusively in another forum, the CFI had total discretion to choose not to exercise its bankruptcy jurisdiction and refrain from determining such threshold question.
The CFA considered that it was at this stage that the public policy interest in holding parties to their agreements was engaged. Should the CFI proceed with the petition and make a ruling on the threshold question, it assumed jurisdiction to decide a question which the parties had otherwise agreed would be determined in another forum.
The CFA was of the view that parties’ agreement for certain disputes to be resolved in another forum would be highly relevant as to whether the CFI should exercise its bankruptcy jurisdiction at all. In the event that the underlying debt was subject to an EJC, unless the Petitioner could show that there were strong reasons, such as the risk of the debtor’s insolvency impacting third parties, the debtor’s reliance on a frivolous defence, or an occurrence of an abuse of process, the Court should normally dismiss the petition.
3. Comment
This decision crystalises the court’s position on the effect of an EJC in the context of bankruptcy and winding up proceedings. Absent strong reasons, the Hong Kong court will not proceed with the petition before the adjudication of the petition debt by the agreed forum. It also underscores the importance attached by the courts to party autonomy.
The case also serves as an important reminder to parties when entering into agreements with EJCs, they should be aware that such clauses will have significant impact on any insolvency proceedings to be commenced in Hong Kong and they may be required to first have the dispute over the underlying debt adjudicated in the agreed forum before commencing insolvency proceedings in Hong Kong.
__________________________
[1] https://legalref.judiciary.hk/lrs/common/ju/ju_frame.jsp?DIS=152321&currpage=T
[2] https://legalref.judiciary.hk/lrs/common/ju/ju_frame.jsp?DIS=137308
[3] https://legalref.judiciary.hk/lrs/common/ju/ju_frame.jsp?DIS=146843
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. Please contact the Gibson Dunn lawyer with whom you usually work, or the following authors in the firm’s Litigation Practice Group in Hong Kong:
Brian W. Gilchrist OBE (+852 2214 3820, bgilchrist@gibsondunn.com)
Elaine Chen (+852 2214 3821, echen@gibsondunn.com)
Alex Wong (+852 2214 3822, awong@gibsondunn.com)
Cleo Chau (+852 2214 3827, cchau@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome
It is no secret among public companies and their counsel that the US Securities and Exchange Commission has steadily adopted a more aggressive stance on cybersecurity controls and disclosure and incident response recordkeeping. SEC Senior Counsel Arsen Ablaev recently highlighted the Commission’s cybersecurity priorities at the annual Incident Response Forum Masterclass. SEC Chair Gary Gensler also emphasized risks in cyber and information security in the March 29 budget hearing with the House Appropriations Committee, and endorsed U.S. President Joe Biden’s request to earmark a record $2.4 billion in funding for the regulator in 2024. Last month saw yet another example of the SEC’s mounting focus on cyber disclosures as an enforcement priority with the announcement that cloud computing company Blackbaud agreed to pay a $3-million civil penalty to settle administrative charges for alleged “materially misleading disclosures” about a 2020 ransomware attack.
As we foreshadowed in our 2023 U.S. Cybersecurity and Data Privacy Outlook and Review, the increase in SEC enforcement resources (e.g., doubling the size of its Crypto Assets and Cyber Unit Ablaev sits in), in combination with the promulgation of cybersecurity risk management, strategy, governance, and incident disclosure rules Ablaev confirmed will be finalized in coming months, signal that cybersecurity will continue to be an area of heightened enforcement activity for the SEC. In light of these developments, it is critical companies take stock of their cyber hygiene policies and incident response protocols, and not only manage cybersecurity risks and prevent attacks, but also respond to them with proper disclosures.
Reproduced with permission from the May 4, 2023 edition of Legaltech News. Copyright 2023 ALM Global Properties, LLC.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding these developments. Please contact the Gibson Dunn lawyer with whom you usually work, any member of the firm’s Privacy, Cybersecurity & Data Innovation practice group, or the authors:
Stephenie Gosnell Handler – Washington, D.C. (+1 202-955-8510, shandler@gibsondunn.com)
David Woodcock – Dallas (+1 214-698-3211, dwoodcock@gibsondunn.com)
Vivek Mohan – Palo Alto (+1 650-849-5345, vmohan@gibsondunn.com)
Mashoka Maimona, an associate working in the firm’s San Francisco office who is admitted only in Ontario, Canada, also contributed to this article.
I. Overview
On May 3, 2023, the U.S. Securities and Exchange Commission (the “SEC” or the “Commission”), by a three-to-two vote, adopted significant amendments (the “Amendments”) to Form PF, the confidential reporting form created in 2011 as part of the Dodd-Frank Act that is intended to provide the Commission and the Financial Stability Oversight Council (“FSOC”) with important data and information about private funds. The Commission, citing Form PF’s historical role in allowing both the Commission and FSOC to monitor systemic risk in the private funds industry, stated that the Amendments will provide regulators with “timely and critical information in times of market stress or volatility” as they attempt to “stem the tides on a potential crisis and help prevent investor harm.”[1]
Form PF is required to be completed and filed by entities which are (i) registered or required to register with the SEC as an investment adviser, (ii) manage one or more private funds, and (iii) together with their related persons, collectively, had at least $150 million in private fund assets under management as of the last day of the most recently completed fiscal year.[2] The Amendments, which introduce two new sections to Form PF, apply to three categories of advisers: (1) hedge fund advisers[3] with at least $1.5 billion in hedge fund assets under management (“Large Hedge Fund Advisers”), (2) investment advisers with at least $150 million in private fund assets under management (“Private Equity Advisers”), and (3) investment advisers with at least $2 billion in private equity fund assets under management (“Large Private Equity Advisers”)[4]. The first new section applies to all Private Equity Advisers, and requires that they make filings within 60 days following the end of any fiscal quarter in which a specified trigger event occurred (the “Quarterly Reporting Window”). The other new section applies only to Large Hedge Fund Advisers, and requires that they make filings as soon as practicable, but no later than 72 hours following certain “significant events that have the potential for broad impacts or investor loss” (the “72 hour Reporting Window”).[5] Finally, the Amendments require that Large Private Equity Advisers report additional data in their annual Form PF filings. The below table summarizes the applicable filing requirements, and each of these changes is described in greater detail below.
|
Triggering Event |
Large Hedge Fund Advisers with $1.5bn+ in hedge fund AUM |
Private Equity Advisers with $150m+ in private fund AUM |
Large Private Equity Advisers with $2bn+ private equity fund AUM |
|
The investment adviser instigates a secondary transaction |
|
File within 60 days of quarter end |
File within 60 days of quarter end |
|
Investors elect to remove the general partner (with or without cause) |
|
File within 60 days of quarter end |
File within 60 days of quarter end |
|
Investors elect to terminate the fund (for any reason) |
|
File within 60 days of quarter end |
File within 60 days of quarter end |
|
Investors elect to terminate the investment period (for any reason) |
|
File within 60 days of quarter end |
File within 60 days of quarter end |
|
10-business day holding period return of fund is less than or equal to 20% of aggregate calculated value |
File within 72 hours |
|
|
|
10 business day change in posted margin, collateral, or equivalent is greater than or equal to 20% of average daily aggregate calculated value during same period |
File within 72 hours |
|
|
|
Fund is in default on a call for margin, collateral or an equivalent that it cannot cover, or adviser determines that fund will not be able to meet such call |
File within 72 hours |
|
|
|
A counterparty to a reporting fund (a) does not meet a call for margin, collateral or equivalent or fails to make any other payment on time and in the form contractually required and (b) the amount involved is greater than 5% of aggregate calculated value |
File within 72 hours |
|
|
|
Termination or material restriction of a reporting fund’s relationship with a prime broker |
File within 72 hours |
|
|
|
There is a “significant disruption or degradation of the reporting fund’s critical operations” |
File within 72 hours |
|
|
|
Fund receives cumulative requests for withdrawals or redemptions equal to at least 50% of the most recent net asset value |
File within 72 hours |
|
|
|
Fund is unable to pay redemption requests |
File within 72 hours |
|
|
|
Fund has suspended redemptions for at least 5 consecutive business days |
File within 72 hours |
|
|
II. Private Equity Advisers are required to file Form PF on a quarterly basis following the occurrence of certain “trigger” events
The Amendments require that all Private Equity Advisers file Form PF in the Quarterly Reporting Window after the end of a fiscal quarter in which either (1) an adviser-led secondary transaction occurred or (2) a fund’s investors elect to remove the general partner, terminate the fund, or terminate the fund’s investment period.[6] Accordingly, Private Equity Advisers are recommended to institute a process wherein they check on a quarterly basis whether a filing is required as a result of such activity.
A. Adviser-Led Secondary Transactions
Private Equity Advisers will be required to file Form PF within the Quarterly Reporting Window after an adviser-led secondary transaction, which the Amendments define as “any transaction initiated by the adviser or any of its related persons that offers private fund investors the choice to: (1) sell all or a portion of their interests in the private fund; or (2) convert or exchange all or a portion of their interests in the private fund for interests in another vehicle advised by the adviser or any of its related persons.”[7] A filing is only required if the transaction is “initiated” by a fund’s Private Equity Adviser (or a related person), which the Commission conceded will require an analysis of the relevant facts and circumstances and noted would generally not include a scenario where the adviser, at the unsolicited request of an investor, participates in a secondary sale of such investor’s fund interest.[8] Importantly, the requirement does not contain exceptions for ordinary-course transactions or situations where the fund’s investors or advisory committee have approved the transaction, as the Commission noted that such approvals, while helpful, “do not always ameliorate investor protection concerns.”[9]
B. Removal of General Partner, Termination of Fund or Termination of Investment Period
Private Equity Advisers will also be required to file Form PF within the Quarterly Reporting Window following: (1) the removal of the adviser or an affiliate thereof as the general partner (or similar control person) of a fund, (2) the election by the fund’s investors to terminate the fund, or (3) the election by the fund’s investors to terminate the fund’s investment period.[10] In each case, the Private Equity Adviser will be required to disclose the effective date of the removal or termination event, as applicable, and a description of such event, and each requirement is triggered upon the adviser receiving notification of the investors’ decision.[11] The Commission noted that only removals or terminations made by the election of the investors would require a filing, but stressed the filing requirement was not limited to “for cause” removals or terminations, noting that such instances are inherently “serious departures from ordinary course operations.”[12]
III. Large Hedge Fund Advisers are required to file Form PF within 72 hours of specified “trigger” events
Under the Amendments, Large Hedge Fund Advisers will be required to file Form PF within the 72 hour Reporting Window following certain triggering events, which are summarized below. As a practical matter, this means that Large Hedge Fund Advisers will need to add a significant number of items to the list of calculations that they run daily in order to determine whether a filing is required.
- Extraordinary Investment Losses. Filing required if, as of any business day, the 10-business day holding period return of a reporting fund is less than or equal to 20% of the reporting fund aggregate calculated value.[13]
- Significant Margin and Default Events. Filing required if (a) during any 10 business days, the change in a reporting fund’s posted margin, collateral, or equivalent is greater than or equal to 20% of such reporting fund’s average daily aggregate calculated value during the same period or (b) the adviser receives notice that a reporting fund is in default on a call for margin, collateral or an equivalent that it cannot cover with additional funds (or determines that such reporting fund will not be able to meet such call).[14]
- Counterparty Default. Filing required if a counterparty to a reporting fund (a) does not meet a call for margin, collateral or equivalent or fails to make any other payment on time and in the form contractually required and (b) the amount involved is greater than 5% of the reporting fund aggregate calculated value.[15]
- Prime Broker Relationship Terminated or Materially Restricted. Filing required following the termination or material restriction of a reporting fund’s relationship with a prime broker.[16]
- Operations Events. Filing required following an “operations event,” which is defined as a “significant disruption or degradation of the reporting fund’s critical operations” (e.g., operations necessary for the investment, trading, valuation, reporting, and risk management of the reporting fund or the operation of the reporting fund in accordance with federal securities laws and regulations).[17]
- Redemptions. Filing required if a reporting fund (a) receives cumulative requests for withdrawals or redemptions equal to at least 50% of the most recent net asset value (after netting against subscriptions or other contributions from investors received and contractually committed), (b) is unable to pay redemption requests, or (c) has suspended redemptions for at least 5 consecutive business days.[18]
The portion of the Amendments covered in sections II and III above will become effective six months after publication of the adopting release in the Federal Register.
IV. Large Private Equity Advisers are required to report additional data as part of their routine Form PF filings
Finally, the Amendments add and amend various questions to Section 4 of Form PF, which is only required to be completed by Large Private Equity Advisers. Most importantly, such advisers will now be required to report whether any reporting fund has effectuated (1) a limited partner clawback (or clawbacks) in excess of an aggregate amount equal to 10% of such fund’s aggregate capital commitments or (2) any general partner clawback, and must provide the reason for such clawback.[19]
The Amendments will also require Large Private Equity Advisers to answer new questions regarding investment strategies of their private equity funds and fund-level borrowing (including with regard to the value of the fund’s borrowings, the types of creditors, and whether the fund can borrow at the fund-level as an alternative or complement to financing of portfolio companies). Additionally, the Amendments will require more granular details regarding certain events of default, bridge financing arrangements (including identifying the institution that provides any bridge loan to a controlled portfolio company), and the geographical breakdown of a fund’s investments (based on a percentage of NAV).[20] Unlike the “trigger” based filing requirements outlined above, changes to Section 4 will become effective one year following publication, and thus should not be relevant for advisers with a December fiscal year end until their annual filings are due in 2025.[21]
V. Analysis
The Amendments will require Private Equity Advisers to add to their quarterly compliance checklists a question as to whether they need to make a filing as a result of a GP-initiated secondary transaction, or a vote of the limited partners to remove the general partner, terminate the fund, or end the investment period early. In addition to paying attention to the other filing requirements described above, Large Hedge Fund Advisers will now need to perform specified calculations on a daily basis (see section III.A. and B. above) and will need to closely monitor redemption requests to determine if they have exceeded 50% of the most recently calculated NAV.
It is not clear what the effect of making a filing under the new requirements will be, but as the Amendments are intended to allow the SEC to monitor systemic risk, such filings will presumably lead to additional questions from the staff, and raise the probability of an SEC inspection.
_____________________________
[1] Press Release, U.S. Securities and Exchange Commission, “Statement on Amendments to Form PF” (May 3, 2023), available at https://www.sec.gov/news/statement/crenshaw-statement-form-pf-050323.
[2] Form PF General Instructions, available at https://www.sec.gov/files/formpf.pdf.
[3] A “hedge fund” generally includes any private fund (other than a securitized asset fund):
(a) with respect to which one or more investment advisers (or related persons of investment advisers) may be paid a performance fee or allocation calculated by taking into account unrealized gains (other than a fee or allocation the calculation of which may take into account unrealized gains solely for the purpose of reducing such fee or allocation to reflect net unrealized losses);
(b) that may borrow an amount in excess of one-half of its net asset value (including any committed capital) or may have gross notional exposure in excess of twice its net asset value (including any committed capital); or
(c) that may sell securities or other assets short or enter into similar transactions (other than for the purpose of hedging currency exposure or managing duration).
Solely for purposes of Form PF, any commodity pool about which you are reporting or required to report on Form PF is categorized as a hedge fund.
For purposes of this definition, long and short positions should not be netted and any borrowings or notional exposure of another person that are guaranteed by the private fund or that the private fund may otherwise be obligated to satisfy should be included.
In general, advisers managing open-ended real asset funds are not considered to be “hedge funds” under Form PF and will not need to make the additional filings included in the Amendments for Large Hedge Fund Advisers. Should you have any questions about the proper filing status of one of your funds, please contact the Gibson Dunn lawyer with whom you usually work, or one of the authors listed herein.
[4] “Regulatory assets under management” corresponds to the same figure reported in the adviser’s Form ADV filing (“RAUM”). “Private fund assets under management” refers to the portion of an adviser’s RAUM attributable to private funds, “private equity fund assets under management” refers to the portion of an adviser’s RAUM attributable to private equity funds, and “hedge fund assets under management” refers to the portion of an adviser’s RAUM attributable to hedge funds.
[5] Press Release, U.S. Securities and Exchange Commission, “Statement on Amendments to Form PF” (May 3, 2023), available at https://www.sec.gov/news/statement/crenshaw-statement-form-pf-050323.
[6] Amendments to Form PF to Require Event Reporting for Large Hedge Fund Advisers and Private Equity Fund Advisers and to Amend Reporting Requirements for Large Private Equity Advisers, SEC Rel. No. IA-6297 (May 3, 2023). Available at https://www.sec.gov/news/press-release/2023-86?utm_medium=email&utm_source=govdelivery.
[7] Id. at 61.
[8] Id.
[9] Id. at 64.
[10] Id. at 64-65.
[11] Id. at 65.
[12] Id. at 67.
[13] Id. at 17. “Reporting fund aggregate calculated value” is defined as follows: Every position in the reporting fund’s portfolio, including cash and cash equivalents, short positions, and any fund-level borrowing, with the most recent price or value applied to the position for purposes of managing the investment portfolio. The reporting fund aggregate calculated value is a signed value calculated on a net basis and not on a gross basis. Where one or more portfolio positions are valued less frequently than daily, the last price used should be carried forward, though a current foreign exchange rate may be applied if the position is not valued in U.S. dollars. It is not necessary to adjust the reporting fund aggregate calculated value for accrued fees or expenses. Reporting fund aggregate calculated value does not need to be subjected to fair valuation procedures. The inclusion of income accruals is recommended but not required; however, the approach should be consistent over time. The reporting fund aggregate calculated value may be calculated using the adviser’s own internal methodologies and conventions of the adviser’s service providers, provided that these are consistent with the information reported internally. “Holding period return” is defined as the cumulative ‘daily rate of return’ over the holding period calculated by geometrically linking the daily rates of return.
[14] Id. at 22-31.
[15] Id. at 31-34.
[16] Id. at 35. Termination events that are set forth in the prime broker (or related) agreement that are isolated to the financial state, activities or other conditions solely of the prime broker do not require a filing.
[17] Id. at 41-48.
[18] Id. at 49-54. While “cumulative” isn’t defined, a filing will be required if, at any time, total withdrawal or redemption requests which have not been granted exceed 50% of NAV.
[19] Id. at 72-78. For purposes of the Amendments, a “limited partner clawback” is defined as “an obligation of a fund’s investors to return all or any portion of a distribution made by the fund to satisfy a liability, obligation, or expense of the fund pursuant to the fund’s governing agreements,” and a “general partner clawback” is defined as “any obligation of the general partner, its related persons, or their respective owners or interest holders to restore or otherwise return performance-based compensation to the fund pursuant to the fund’s governing agreements.”
[20] Id. at 79-83.
[21] Id. at 87-88.
Gibson Dunn’s lawyers are available to assist with any questions you may have regarding the issues and considerations discussed above. Please contact the Gibson Dunn lawyer with whom you usually work in the firm’s Investment Funds practice group, or the following authors:
Gregory Merz – Washington, D.C. (+1 202-887-3637, gmerz@gibsondunn.com)
Lauren Cook Jackson – Washington, D.C. (+1 202-955-8293, ljackson@gibsondunn.com)
Shannon Errico – New York (+1 212-351-2448, serrico@gibsondunn.com)
Robert Harrington – New York (+1 212-351-2608, rharrington@gibsondunn.com)
Investment Funds Group Contacts:
Jennifer Bellah Maguire – Los Angeles (+1 213-229-7986, jbellah@gibsondunn.com)
Albert S. Cho – Hong Kong (+852 2214 3811, acho@gibsondunn.com)
Candice S. Choh – Los Angeles (+1 310-552-8658, cchoh@gibsondunn.com)
John Fadely – Singapore/Hong Kong (+65 6507 3688/+852 2214 3810, jfadely@gibsondunn.com)
A.J. Frey – Washington, D.C./New York (+1 202-887-3793, afrey@gibsondunn.com)
Shukie Grossman – New York (+1 212-351-2369, sgrossman@gibsondunn.com)
James M. Hays – Houston (+1 346-718-6642, jhays@gibsondunn.com)
Kira Idoko – New York (+1 212-351-3951, kidoko@gibsondunn.com)
Eve Mrozek – New York (+1 212-351-4053, emrozek@gibsondunn.com)
Roger D. Singer – New York (+1 212-351-3888, rsinger@gibsondunn.com)
Edward D. Sopher – New York (+1 212-351-3918, esopher@gibsondunn.com)
William Thomas, Jr. – Washington, D.C. (+1 202-887-3735, wthomas@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome
On May 2, 2023, in a case challenging the fairness of a merger between a cloud security startup and a Boston-based cybersecurity company, the Delaware Court of Chancery denied a motion to dismiss breach of fiduciary duty claims relating to a drag-along merger despite plaintiff-investors’ explicit covenant not to sue over a drag-along sale, including by raising claims of fiduciary duty breach. In a decision by Vice Chancellor J. Travis Laster, the court found that, as a matter of public policy, a covenant not to sue cannot shield defendants from tort liability for intentional harm. This decision follows a March 9, 2023 decision denying a motion to dismiss the same complaint for failure to state a claim. Although the court’s decision may leave directors and controlling stockholders unable to fully protect themselves from bad faith breach of fiduciary duty claims after a drag-along transaction, it does provide some guidance for controllers relying on fiduciary claim waivers.
Background
The plaintiffs in New Enterprise Associates 14, L.P. v. Rich[1] are investment funds managed by venture capital firms, each holding an interest in cloud-security company Fugue, Inc. In early 2021, after an unsuccessful sale process and receiving plaintiffs’ indication of unwillingness to provide additional financial support, the company’s management concluded that a recapitalization led by George Rich was the only viable option for raising necessary capital. As a condition of his investment, Rich required the plaintiffs to enter into a voting agreement that, like typical NVCA-style voting agreements, included a covenant not to sue for a breach of fiduciary duty arising from a drag-along transaction. Notably, the plaintiffs declined an offer to participate in the recapitalization.
Following the recapitalization, Rich and his associates held a controlling interest in the company and controlled the board. In June 2021, the company was contacted by a potential acquirer. Soon after the company’s independent directors resigned in July 2021, the board approved two equity issuances. First, the board issued additional preferred stock to many of the original recapitalization investors at the same distressed price per share as their original investment. According to the plaintiffs, the company both ignored the right of first offer (the “ROFO”) of the plaintiffs found in a side letter signed in connection with the recapitalization and failed to deliver a notice of stockholder action to the plaintiffs under Section 228(e) of the Delaware General Corporation Law. Second, the board approved an issuance of stock options—mostly to themselves—with vesting provisions that accelerated upon a change of control. The plaintiffs allege these transactions were interested and not disclosed.
Shortly after these issuances, the company’s management negotiated a merger with Snyk Limited, a private cybersecurity company. The plaintiffs were asked to join the merger, but refused when Rich and another director refused to attest that they had not communicated with the acquirer regarding a potential transaction before the recapitalization. After the merger closed, the plaintiffs learned of the interested transactions and sued the company, its board of directors, and affiliated entities for breach of fiduciary duty. Because the merger had extinguished the plaintiffs’ standing to bring derivative claims, they instead filed a direct breach of fiduciary duty suit arguing that the merger consideration was inadequate since it did not include the valuable derivative claims that the company had against the defendants for the interested transactions.
In New Enterprise I, the Court of Chancery found that the plaintiffs established standing under Primedia because they had viable underlying derivative claims against the defendants (fiduciary claims regarding the interested transactions as well as disclosure claims), the derivative claims were material (almost 10% of the transaction proceeds), and it was unlikely the acquirer would assert the claims.[2] The court also found that the plaintiffs had adequately stated a claim for breach of fiduciary duty because the defendants were alleged to have a conflict of interest with respect to the merger which extinguished possible derivative causes of action against themselves. The court initially withheld ruling on the defendants’ alternative argument that the drag-along covenant foreclosed the plaintiffs from bringing suit to challenge the merger.
Ruling
On May 2, 2023, in New Enterprise II,[3] the court addressed the defendants’ argument regarding the drag-along covenant. In a detailed discussion of Delaware law, Vice Chancellor Laster clarified that a covenant not to sue for breach of fiduciary duty was not, on its own, facially invalid. Recognizing that Delaware corporate law allows some degree of private fiduciary tailoring, the court noted that “stockholders can agree to more constraints on their ability to exercise stockholder-level rights than corporate planners can impose through the charter or bylaws.” The court also noted that the covenant was clear, specific, and limited to contractually outlined criteria for drag-along sales; it was part of a bargained for-exchange that included the recapitalization; and the parties were sophisticated repeat players who understood its application (one of the plaintiffs also being a member of the NVCA). Nonetheless, the court held that for reasons of Delaware public policy regarding contracts, the covenant could not “insulate the defendants from tort liability based on intentional wrongdoing,” which the court found to be adequately pled.
Uncertain Path Forward
The court’s decision allows for some fiduciary tailoring among stockholders, including through covenants not to sue. The court stated that the covenant could provide protection from claims that “the defendants engaged in self-interested transactions but believed in good faith that the transactions were not contrary to the best interests of the Company” or claims that “the defendants engaged in the self-interested transactions with reckless disregard for the best interests of the Company.”
Moreover, the case at hand presented unique facts, as the plaintiffs agreed to the drag-along in the context of the recapitalization, but allegedly were not informed of subsequent interested transactions that altered their rights. The acts of intentional harm underlying the court’s decision to deny the motion to dismiss did not occur directly as part of the drag-along and became choate only when the plaintiffs learned of the interested transactions and breaches of ROFO protections for which they had negotiated in the original recapitalization. This decision may leave practitioners wary of relying on covenants not to sue in connection with drag-along transactions. It remains to be seen how readily allegations of intentional torts, such as bad faith breach of fiduciary duty, that arise solely from a drag transaction among sophisticated parties (and without incriminating prior conduct similar to the alleged interested transactions) will overcome a similar NVCA-style covenant not to sue.
An immediate and practical takeaway for corporate practitioners is to ensure that contractual and statutory notices (whether preemptive rights notices or Section 228(e) notices) are properly delivered, so that stockholders have access to their negotiated and statutory rights in connection with the lead-up to and execution of the drag transaction.
_________________________
[1] See New Enter. Assocs. 14, L.P. v. Rich (“New Enterprise I”), 2023 WL 2417271 (Del. Ch. Mar. 9, 2023).
[2] Id. at *29–40 (citing In re Primedia, Inc. S’holders Litig., 67 A.3d 455 (Del. Ch. 2013)).
[3] New Enter. Assocs. 14, L.P. v. Rich (“New Enterprise II”), 2023 WL 3195927 (Del. Ch. May 2, 2023).
The following Gibson Dunn lawyers prepared this client alert: Benyamin S. Ross, Marshall R. King, Marina Szteinbok, Mark Goldman, Mark H. Mixon, Jr., Adrian Melendez-Cooper, Sam Shapiro, and Valy Menendez.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding the issues discussed in this update. Please contact the Gibson Dunn lawyer with whom you usually work, any member of the firm’s Emerging Companies, Mergers and Acquisitions, Private Equity, or Securities Litigation practice groups, or the following authors and practice leaders and members:
Benyamin S. Ross – Los Angeles (+1 213-229-7048, bross@gibsondunn.com)
Marshall R. King – New York (+1 212-351-3905, mking@gibsondunn.com)
Emerging Companies Group:
Chris W. Trester – Palo Alto (+1 650-849-5212, ctrester@gibsondunn.com)
Mergers and Acquisitions Group:
Robert B. Little – Dallas (+1 214-698-3260, rlittle@gibsondunn.com)
Saee Muzumdar – New York (+1 212-351-3966, smuzumdar@gibsondunn.com)
Private Equity Group:
Richard J. Birns – New York (+1 212-351-4032, rbirns@gibsondunn.com)
Ari Lanin – Los Angeles (+1 310-552-8581, alanin@gibsondunn.com)
Michael Piazza – Houston (+1 346-718-6670, mpiazza@gibsondunn.com)
Securities Litigation Group:
Monica K. Loseman – Denver (+1 303-298-5784, mloseman@gibsondunn.com)
Brian M. Lutz – San Francisco/New York (+1 415-393-8379/+1 212-351-3881, blutz@gibsondunn.com)
Craig Varnen – Los Angeles (+1 213-229-7922, cvarnen@gibsondunn.com)
© 2023 Gibson, Dunn & Crutcher LLP
Attorney Advertising: The enclosed materials have been prepared for general informational purposes only and are not intended as legal advice. Please note, prior results do not guarantee a similar outcome