FDA Publishes Proposed Rule on Front-of-Package Labeling – What You Need to Know
Client Alert | January 15, 2025
Comments on the Front-of-Package Proposed Rule must be submitted to FDA by May 16, 2025.
On January 14, 2025, the U.S. Food and Drug Administration (FDA) published a much-anticipated proposed rule that, if finalized, will require front-of-package (FOP) nutrition labeling for foods (FOP Proposed Rule). The proposed rule is the culmination of almost two decades of consideration by FDA and Congress of whether and in what form to require an abbreviated FOP nutrition disclosure on food packages, with the stated goal of aiding consumers in making healthier choices.[2] FDA’s development of the approach set forth in the proposed rule included focus groups and experimental testing of various formats, including a Guideline Daily Amount (GDA) format resembling the industry-developed Facts Up Front (FUF) scheme.[3]
The proposed rule contains provisions that have been hotly contested by both the food industry and health advocates and is expected to face significant administrative and constitutional challenges if finalized by the incoming Trump administration. Comments on the Front-of-Package Proposed Rule should be submitted to Docket No. FDA-2024-N-2910 by the deadline of 120 days after the publication, on May 16, 2025.
Here are five things to know about the FOP Proposed Rule:
- Introducing the “Nutrition Info” Panel: The FOP Proposed Rule introduces a new black-and-white “Nutrition Info” panel that will appear on the front of food packages. The Nutrition Info panel, shown below, provides the serving size of the food and the per-serving percent Daily Value (DV) of three nutrients: saturated fat, sodium, and added sugars. Characterizations of each of these three nutrients will be included as “Low” (5% DV or less), “Med” (6% to 19% DV), or “High” (20% DV or more). The panel will also include an attribution to “FDA.gov,” intended to increase consumer credibility and trust.[4]
 |
FDA also allows for a smaller version of the Nutrition Info panel for foods in smaller packages with less than 40 square inches to present the Nutrition Facts label in tabular fashion. This smaller format includes only the “Low,” “Med,” or “High” characterizations for the three nutrients to limit, without the serving size (or disclosure that the characterization is based on serving size) or percent DVs for each:[5]
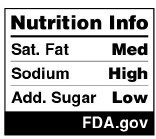 |
Similar to FDA’s current regulations for Nutrition Facts, FDA proposes to exempt foods in packages with a total surface area of less than 12 square inches from bearing the Nutrition Info box.[6] The FOP Proposed Rule also includes special formats for certain types of foods, including packages that contain separately packaged foods intended to be eaten individually, such as variety packs; foods with Nutrition Facts labeling for two or more population groups, for both per serving and per individual unit amounts, and for the food “as packaged” and “as prepared;” foods sold from bulk containers; and, game meats.[7]
- “High,” “Med,” “Low”: Following its testing of various schemes, FDA has chosen to propose a black-and-white Nutrition Info panel (i.e, rather than a red/yellow/green traffic light schema) that includes characterizations of the levels of nutrients to limit, rather than presenting plain numerical data. FDA justified this approach based on scientific literature showing that consumers struggled to understand numeric values in current nutrition labeling when making choices about food.[8] FDA stated that its “Low” and “High” characterizations are consistent with its historical approach for thresholds for “low” and “high” claims for sodium and saturated fats. While there are no current regulations on “medium” nutrient content claims, the agency is establishing “Med” to refer to products that fall between the “Low” and “High” categories.[9] Consistent with this approach, FDA also proposes amendments to its regulations to provide for low sodium and low saturated fat nutrient content claims in line with current nutrition science and the updated Daily Reference Value (DRV) for sodium in the 2016 Nutrition Facts label final rule.[10]
- Who is Subject to the FOP Proposed Rule?: FDA proposes to require all foods currently marketed to people ages 4 and older – the population FDA considers to constitute the general population for nutrition labeling – to comply with the Nutrition Info box requirements, unless specific exemptions apply.[11] The proposed rule includes exemptions for, among others, foods in packages with a total surface area of less than 12 square inches; packages marketed as gifts containing a variety or assortment of foods; and, unit containers in multiunit retail food packages.[12]
- When Does the FOP Nutrition Labeling Requirement Go into Effect?: If the proposed rule is finalized as published, it would require manufacturers to add a Nutrition Info box to packaged food products three years after the final rule’s effective date (for businesses with $10 million or more in annual food sales) and four years after the effective date (for businesses with less than $10 million in annual food sales). However, before the rule can be finalized, FDA must review any comments on the proposed rule and issue a final rule. This process can take anywhere from one to several years, depending on the number and nature of comments received and agency (and broader administration) priorities.
- Making America Healthy Again?: Similar to other recently issued FDA guidance and regulations, it is unclear whether the FOP Proposed Rule will be finalized following the change in administration. Nutrition and transparency in food labeling are also priorities of anticipated leadership for FDA and the U.S. Department of Health and Human Services (HHS) under the incoming Trump administration. It remains to be seen whether FOP nutrition labeling will feature as part of FDA’s food regulatory priorities moving forward.
Interested parties should submit comments to the docket. The FOP Proposed Rule is scheduled to be published in the Federal Register on January 16, 2025. Comments on the FOP Proposed Rule should be submitted to Docket No. FDA-2024-N-2910 by the deadline of 120 days after the publication, on May 16, 2025. Gibson Dunn is prepared to help interested parties consider the implications of this proposed rule, if finalized, including through regulatory counseling, FDA and legislative engagement, and litigation.
[1] FDA, “Food Labeling: Front-of-Package Nutrition Information,” available at https://www.federalregister.gov/public-inspection/2025-00778/food-labeling-front-of-package-nutrition-information (last accessed Jan. 14, 2025) (FOP Proposed Rule). The FOP Proposed Rule is scheduled to be published in the Federal Register on Thursday, January 16, 2025.
[2] See id., Part III.B-D.
[3] See id., Part III.D.2-3.
[4] Id., Part I.A, V.B, V.B.2, V.B.5.
[5] Id. Part V.E.5.
[6] Id. Part V.F.2.
[7] Id. Part V.E.1-4, 6-7.
[8] Id., Part III.A, D.2-3.
[9] Id., Part IV.B.3.
[10] Id., Part V.G.
[11] Id., Part V.A.
[12] Id., Part V.F.1-4.
[13] Regulations.gov, Docket No. FDA-2024-N-2910.
Gibson Dunn’s lawyers are available to assist in addressing any questions you may have regarding the issues discussed in this update. Please contact the Gibson Dunn lawyer with whom you usually work, the authors, or any leader or member of the firm’s FDA & Health Care practice group:
Gustav W. Eyler – Washington, D.C. (+1 202.955.8610, geyler@gibsondunn.com)
Katlin McKelvie – Washington, D.C. (+1 202.955.8526, kmckelvie@gibsondunn.com)
John D. W. Partridge – Denver (+1 303.298.5931, jpartridge@gibsondunn.com)
Jonathan M. Phillips – Washington, D.C. (+1 202.887.3546, jphillips@gibsondunn.com)
Carlo Felizardo – Washington, D.C. (+1 202.955.8278, cfelizardo@gibsondunn.com)
Wynne Leahy – Washington, D.C. (+1 202.777.9541, wleahy@gibsondunn.com)
© 2025 Gibson, Dunn & Crutcher LLP. All rights reserved. For contact and other information, please visit us at www.gibsondunn.com.
Attorney Advertising: These materials were prepared for general informational purposes only based on information available at the time of publication and are not intended as, do not constitute, and should not be relied upon as, legal advice or a legal opinion on any specific facts or circumstances. Gibson Dunn (and its affiliates, attorneys, and employees) shall not have any liability in connection with any use of these materials. The sharing of these materials does not establish an attorney-client relationship with the recipient and should not be relied upon as an alternative for advice from qualified counsel. Please note that facts and circumstances may vary, and prior results do not guarantee a similar outcome.